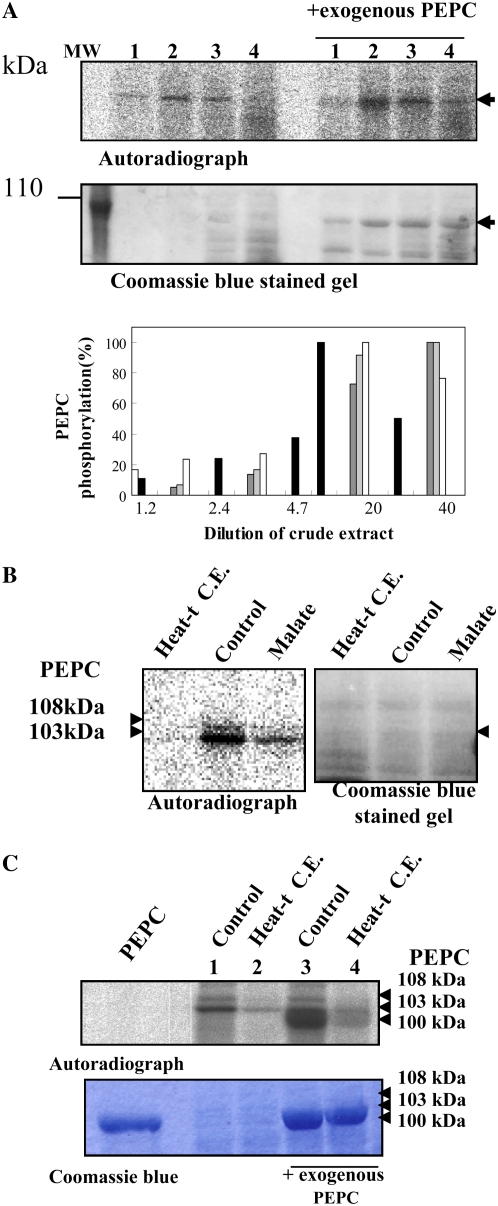
Figure 5.
A, Effect of nondesalted extract concentration on in vitro PEPC kinase activity. The phosphorylation assays were performed at different crude extract concentrations from whole dry seeds, at 30°C during 45 min, in the presence of 1 mm EGTA, the presence or absence of a purified, nonphosphorylated sorghum leaf PEPC as an exogenous substrate (0.2 units), and the remaining components of the phosphorylation assay. Lanes 1 to 4 correspond to 24, 48, 242, and 485 μg of protein that contained 0.005, 0.01, 0.05, and 0.1 units of endogenous PEPC in a final volume of 120 μL. MW, Molecular mass marker. The histogram shows the results of different experiments (different gray intensities), and phosphorylation was quantified by the program Image J.130. B, Effect of a heat-treated nondesalted seed extract and malate on PEPC phosphorylation. The phosphorylation assays were performed in the standard conditions described above (control) using nondesalted crude extracts from dry seeds (40 μg of total protein containing 0.01 units of endogenous PEPC in 120 μL) and in the presence of an aliquot (200 μg of total protein) of the supernatant of denatured (2 min at 90°C) and centrifuged crude extracts (removing most proteins including PEPC) prepared from dry seeds (Heat-t C.E.) or l-malate (malate) at a concentration of 0.6 mm. After the phosphorylation assay, proteins were separated by SDS-PAGE (10% acrylamide) and analyzed by autoradiography. C, The experiment was performed as indicated in B, with denatured and centrifuged crude extracts (Heat-t C.E.) from 11-DPA seeds. The sorghum leaf PEPC was added as a marker (Coomassie Brilliant Blue; PEPC). Phosphorylated seed PEPC bands (108 and 103 kD) and the sorghum leaf PEPC band (100 kD) are clearly detected on the autoradiograph. [See online article for color version of this figure.]