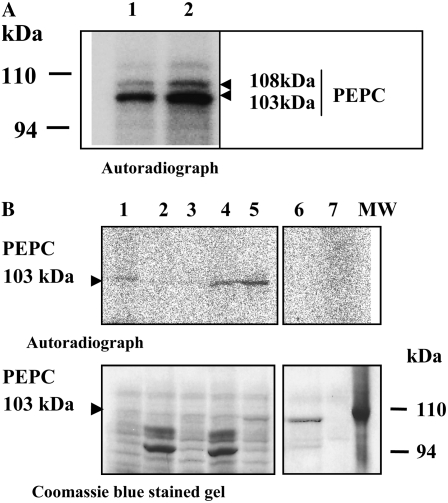
Figure 6.
The inhibitory effect related to crude extract concentration was abolished by desalting the crude extract or by a preincubation of the denatured crude extracts with MDH. A, Phosphorylation assays were performed using the standard conditions and desalted crude extracts (filtration through Sephadex G-25) from whole dry seeds. Lane 1, 0.05 PEPC units per 242 μg of proteins; lane 2, 0.1 PEPC units per 484 μg of proteins. B, The phosphorylation assays were performed using the conditions described for Figure 5A, with nondesalted crude extracts of whole dry seeds (48 μg of protein containing 0.01 units of PEPC in 120 μL). Lane 1, crude extract; lane 2, crude extract + an aliquot of the supernatant of heat-treated nondesalted crude extract (262 μg of total protein); lane 3, crude extract + 4 mm l-malate; lane 4, crude extract + an aliquot of the supernatant of heat-treated nondesalted crude extract (262 μg of protein) treated with 50 units of MDH and 4 mm NAD+; lane 5, crude extract + a purified nonphosphorylated sorghum leaf PEPC as exogenous substrate (0.05 units); lane 6, nonphosphorylated sorghum leaf PEPC (0.15 units); lane 7, components of the ADP scavenging system (adenylate kinase inhibitor, phosphocreatine, and creatine phosphokinase). The phosphorylation assays were performed in the presence of 1 mm EGTA at 30°C during 45 min. After denaturation, the proteins were separated by SDS-PAGE (11% acrylamide) and analyzed by autoradiograph. MW, Molecular mass markers.