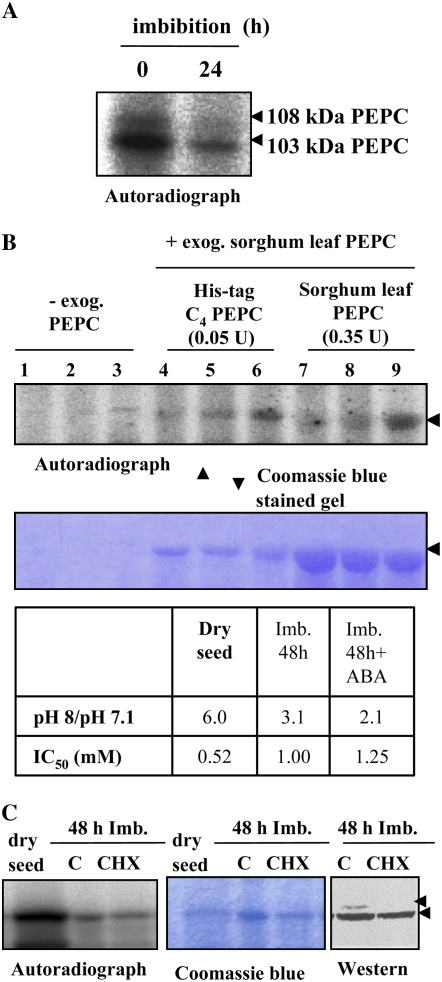
Figure 8.
A, Degradation of PEPC kinase during seed imbibition. The phosphorylation assay was performed using desalted crude extracts from deembryonated barley seeds imbibed in water for 24 h at room temperature. B, ABA and CHX affect PEPC kinase activity during imbibition and germination. Seeds were allowed to imbibe in water (lanes 1, 4, and 7), 40 μm ABA (lanes 2, 5, and 8), or 40 μm ABA and 25 μm CHX (lanes 3, 6, and 9) for 78 h at room temperature. In addition to the endogenous PEPC, this experiment was performed using two different preparations of exogenous PEPC in the phosphorylation assays: purified nonphosphorylated or recombinant (His-tag C4 PEPC) sorghum leaf PEPC. Proteins were separated by SDS-PAGE (11% acrylamide) and analyzed by autoradiography. The table shows the in vivo phosphorylation state of PEPC as determined by the ratio between PEPC activity at pH 8 and pH 7.1 and by the malate test (IC50). The arrow indicates 103-kD PEPC. C, Seeds were imbibed for 72 h in the absence (C) or presence (CHX) of 25 μm CHX. Phosphorylation assays were carried out as above in the absence of exogenous PEPC. Transferred proteins on nitrocellulose membranes (western) were incubated with polyclonal anti-PEPC IgGs from sorghum leaves (30 μg per 20 mL of incubation medium), and immunolabeled proteins were detected by a horseradish peroxidase assay. The arrows indicate 103- and 108-kD PEPC subunits. [See online article for color version of this figure.]