Table 3.
Wittig Reaction of Various Aldehydes with 4a
 | ||||
|---|---|---|---|---|
| entry | aldehyde | reaction time (h) | product | Yield
(%)b |
| 1 |
|
4 |
14 E:Z = 1.7:1 |
82 |
| 2 |

|
12 |

15 E:Z = 4.6:1 |
87 |
| 3 |
|
10 |

16 E:Z = 7.6:1 |
63 |
| 4 |

|
7 |

17 E:Z = 2.9:1 |
72
(65)c |
| 5 |
|
5 |
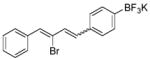
18 E:Z = 4.0:1 |
76 |
| 6 |
|
11 |

19 E:Z = 1.5:1 |
98 |
| 7 |
|
1 |

20 E:Z = 2.8:1 |
92
(87)c |
| 8 |

|
2 |

21 E:Z = 1:1.7 |
83
(94)c |
All reactions were carried out on 0.1 mmol scale in 800 μL of DMSO-d6 and sonicated for 20 min.
Yield is given for the isolated products and the E/Z ratios were determined by 1H NMR.
Reaction was performed on 1 mmol scale in 3 mL of DMF.
