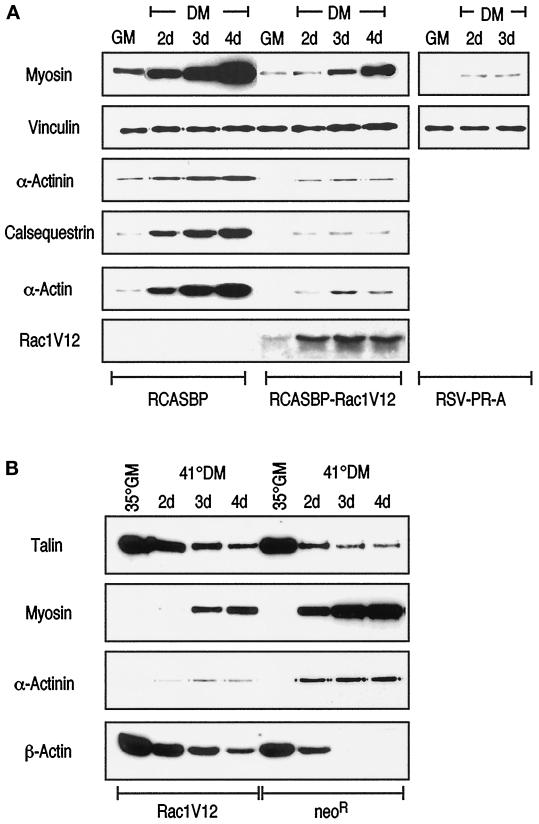
Figure 6.
Rac1V12 inhibits accumulation of muscle-specific proteins in myotubes. Western blot analysis of cell extracts from QMb infected with RCASBP, RCASBP-Rac1V12, or RSV-PR-A retroviruses and cultured in DM for 2, 3, and 4 d (A) and polyclonal populations of QMb-LA29 stably transfected with expression vectors encoding a neomycin-resistance gene (neoR) or Rac1V12 cultured in GM at the permissive temperature (35°C) or in DM at the restrictive temperature (41°C) for 2, 3, and 4 d (B). Cell extracts were then analyzed for expression of cytoskeletal and muscle-specific proteins by immunoblotting with specific antibodies, as indicated. Probing of the blots with antibodies to cytoskeletal and muscle-specific proteins shows that the accumulation of myofibrillar proteins such as myosin, α-actinin, and calsequestrin during in vitro maturation is highly reduced in myotubes expressing Rac1V12 compared with controls. Conversely, the reduction of cytoskeletal proteins such as β-actin and talin, which usually accompany myotube maturation, is partly inhibited by the presence of Rac1V12. The data shown are representative of three independent experiments.