TABLE 2.
Synthesis of 3-Iodoindoles.a
| entry | alkyne | time (h) | product | isolated yield (%) | ||
|---|---|---|---|---|---|---|
| 1 |
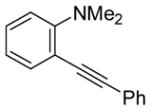
|
2 | 2 |
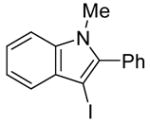
|
29 | 100 |
| 2 |
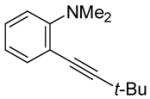
|
3 | 2 |
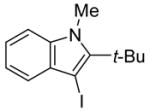
|
30 | 96 |
| 3 |
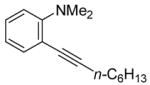
|
4 | 2 |
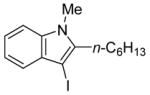
|
31 | 94 |
| 4 |
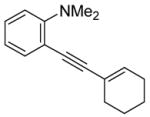
|
5 | 2 |
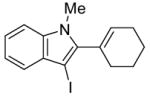
|
32 | 85 |
| 5 |
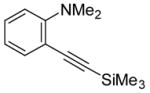
|
6 | 4 |
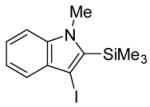
|
33 | ? |
| 6 |
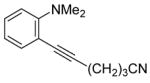
|
7 | 4 |
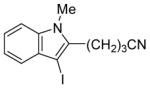
|
34 | 86 |
| 7 |
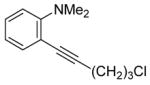
|
8 | 4 |
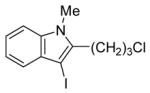
|
35 | 73 |
| 8 |
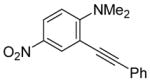
|
10 | 0.5 |
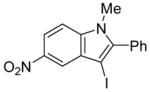
|
36 | 100 |
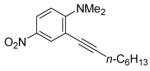
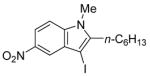
| 9 |
|
11 | 0.5 |
|
37 | 95 |
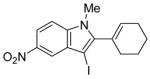
| 10 |
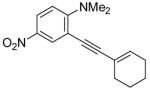
|
12 | 0.5 |
|
38 | 100 |
| 11 |
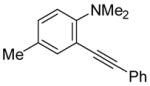
|
14 | 0.5 |
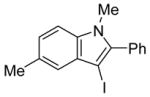
|
39 | 100 |
| 12 |
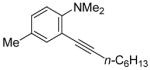
|
15 | 0.5 |
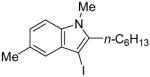
|
40 | 100 |
| 13 |
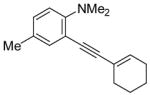
|
16 | 0.5 |
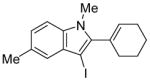
|
41 | 98 |
| 14 |
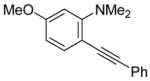
|
18 | 2 |
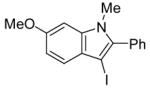
|
42 | 84 |
| 15 |
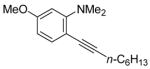
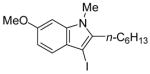
|
19 | 2 |
|
43 | 84 |
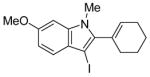
| 16 |
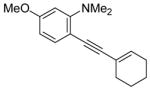
|
20 | 2 |
|
44 | 100 |
| 17 |
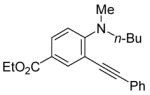
|
22 | 0.5 |
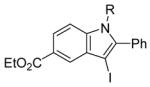
R = n-Bu (45)R = Me (46) |
98(72:28) b(45:46) | |
| 18 |
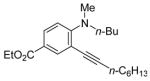
|
23 | 0.5 |
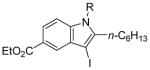
R = n-Bu (47)R = Me (48) |
84(62:38) b(47:48) | |
| 19 |
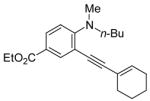
|
24 | 0.5 |
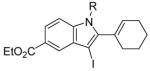
R = n-Bu (49)R = Me (50) |
92(66:34) b(49:50) | |
| 20 |
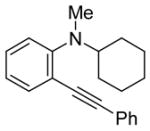
|
26 | 2 |
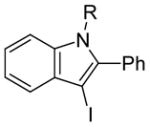
R = Me (27) |
50(90:10) b(27:51) | |
| 21 |
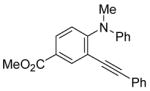
|
28 | 1 |
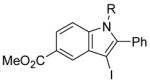
R = Ph (52)R = Me (27) |
80(100:0) b(52:27) |
All reactions were run with 0.25 mmol of the alkyne and 2 equiv of I2 in 5 mL of CH2Cl2 at 25 °C, followed by the addition of 5 mL of satd aq Na2S2O3 to remove the excess I2.
Ratio of the products shown immediately below.
