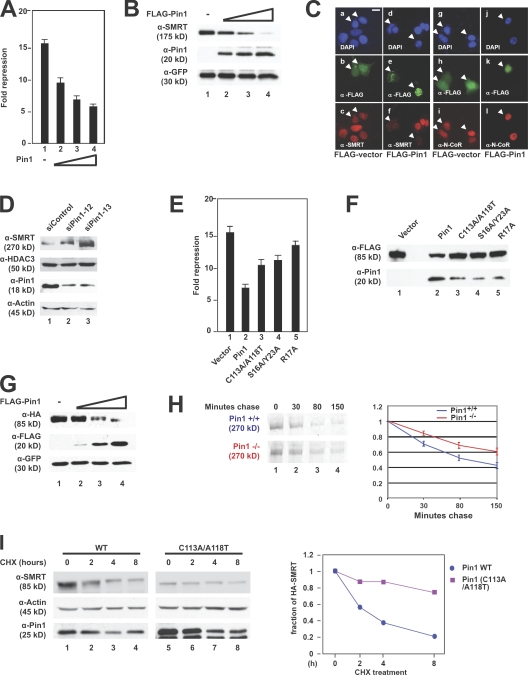
Figure 2.
Pin1 down-regulates SMRT protein levels. (A) Overexpression of Pin1 relieves SMRT repression. CV-1 cells were transiently transfected with Gal4-SMRT (1,050–1,823), increasing amounts of Pin1, and a thymidine kinase–luciferase reporter. Luciferase activity was measured 36–48 h after transfection; all experiments were performed in triplicate. Error bars represent ±SD. (B) Overexpression of Pin1 has a negative effect on SMRT steady-state levels. SMRT (1,012–2,507), FLAG-Pin1, and GFP were cotransfected into CV-1 cells. WCEs were subjected to immunoblotting with the indicated antibodies. (C) Ectopic expression of Pin1 affects endogenous SMRT levels in MCF-7 cells. Cells were transfected with FLAG-Pin1 or FLAG-vector and immunostained with the indicated antibodies 24 h after transfection. (top) DAPI staining; (middle) α-FLAG staining; (bottom) SMRT or N-CoR staining. Arrowheads indicate transfected cells. Bar, 5 μm. (D) siRNA-mediated knockdown of Pin1 increases SMRT protein levels. HeLa cells were transfected with either a control oligonucleotide or one of two Pin1-targeting siRNAs. Cells were harvested 72 h later, and WCEs were subjected to immunoblotting with the indicated antibodies. (E) Pin1 mutants lose the ability to relieve SMRT repression. CV-1 cells were transiently transfected with Gal4-SMRT (1,050–1,823), Pin1 WT or mutants, and a thymidine kinase–luciferase reporter. Luciferase activity was measured 36–48 h later; all experiments were performed in triplicate. Error bars represent ±SD. (F) Pin1 mutants lose the ability to down-regulate SMRT levels. CV-1 cells were cotransfected with FLAG-SMRT (1,178–1,823) and HA-tagged Pin1 WT or mutants. WCEs were subjected to immunoblotting with the indicated antibodies. (G) Exogenous overexpression of Pin1 in Pin1−/− MEFs decreases SMRT levels. HA-SMRT (1,178–1,823), FLAG-Pin1, and GFP were cotransfected into Pin1−/− MEFs, and immunoblotting was performed using the indicated antibodies. (H) SMRT half-life is increased in Pin1−/− MEFs. Pin1+/+ and Pin1−/− MEFs were pulse labeled with [35S]methionine and [35S]cysteine and chased with cold growth medium as described in Materials and methods. WCEs were prepared and subjected to immunoprecipitation, and protein levels were detected by autoradiography. Left, 35S autoradiography; right, quantification of immunoprecipitated 35S-labeled SMRT. Error bars represent ±SD. (I) SMRT destabilization requires Pin1 isomerase activity. HeLa cells stably expressing shRNA against Pin1 were transfected with SMRT (1,178–1,823) and either WT Pin1 or Pin1 (C113A/A118T) and treated with 100 μg/ml CHX for the indicated times, and immunoblots were performed using the indicated antibodies. Left, immunoblot; right, quantification of SMRT levels normalized to actin levels.