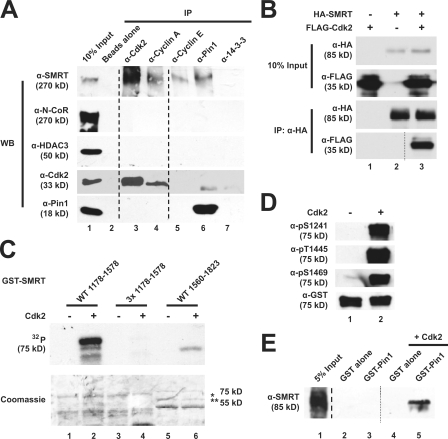
Figure 5.
Cdk2 interacts with and phosphorylates SMRT to generate Pin1-binding sites. (A) Endogenous SMRT coimmunoprecipitates with Cdk2 in HeLa nuclear extracts. Coimmunoprecipitations were performed as described in Fig. 1 D using the indicated antibodies (top) followed by immunoblotting with the indicated antibodies (left). Unrelated lanes were removed. (B) Exogenous Cdk2 and SMRT interact. HA-SMRT (1,178–1,823) and FLAG-Cdk2 were cotransfected into HeLa cells. Coimmunoprecipitations were performed as in Fig. 1 A and immunoblotted with the indicated antibodies. (C) Cdk2 phosphorylates SMRT in vitro. Purified GST-SMRT proteins (WT 1,178–1,578, 3× mutant 1,178–1,578, and WT 1,560–1,823) were incubated with purified Cdk2 and [32P]ATP. Samples were resolved by SDS-PAGE and transferred to a polyvinylidene difluoride membrane before autoradiography. Top, autoradiography; bottom, Coomassie staining; *, GST-SMRT (1,178–1,578); **, GST-SMRT (1,560–1,823). (D) Cdk2 phosphorylates S1241, T1445, and S1469 of SMRT. In vitro kinase assays were performed as aforementioned using GST-SMRT (1,178–1,588), Cdk2, and unlabeled ATP. Immunoblotting was performed with the indicated antibodies. (E) Cdk2 phosphorylation of SMRT generates Pin1-binding sites. His6-SMRT (1,178–1,823) was subjected to in vitro kinase assays as described in D. After kinase reactions, samples were subjected to GST pull downs with GST–Pin1. Immunoblotting was performed with the indicated antibodies. Unrelated lanes were removed.