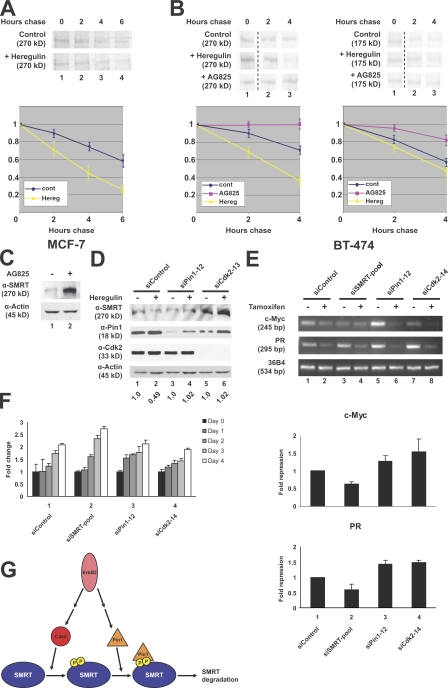
Figure 7.
ErbB2 attenuates SMRT levels in breast cancer cells. (A) SMRT half-life in MCF-7 cells. Cells were pulse labeled with [35S]methionine and [35S]cysteine and chased with cold growth medium. Additionally, cells were treated with 100 ng/ml heregulin or a vehicle control for 30 min before labeling. Top, 35S autoradiography; bottom, quantification of immunoprecipitated 35S-labeled SMRT. (B) SMRT half-life in BT-474 cells. Experiments were performed according to the description in A with the addition of 0.1μM AG825 treatment. Unrelated lanes were removed. Left, endogenous SMRT; right, transfected HA-SMRT (1,012–2,507); top, 35S autoradiography; bottom, quantification of immunoprecipitated 35S-labeled SMRT. (C) AG825 treatment increases SMRT protein levels. BT-474 cells were treated with 0.1 μM AG825 or vehicle control for 48 h. WCEs were immunoblotted with the indicated antibodies. (D) ErbB2 controls SMRT levels through a Pin1- and Cdk2-dependent pathway. BT-474 cells were transfected with siRNAs for 24 h followed by treatment with 100 ng/ml heregulin for 48 h. WCEs were subjected to immunoblotting with the indicated antibodies. Quantification of SMRT levels normalized to actin is shown below the blot; quantification was performed in Photoshop. (E) SMRT is required for ERα-dependent repression in response to tamoxifen. BT-474 cells were transfected with siRNAs for 48 h followed by treatment with 100 nM 4-hydroxytamoxifen for 72 h. Total RNA was harvested followed by cDNA generation. RT-PCR was performed using primers targeting the indicated genes. Top, ethidium bromide staining; bottom, quantification of three sets of experiments for each gene. (F) SMRT is required for tamoxifen-dependent inhibition of cell proliferation. BT-474 cells were transfected with siRNAs as in E. 48 h after transfection, cells were counted, split evenly onto plates, and treated with 100 nM 4-hydroxytamoxifen for the indicated times. Proliferation was measured every 24 h. Error bars represent ±SD. (G) Model of SMRT regulation by Pin1 and Cdk2. SMRT is phosphorylated by Cdk2–cyclin A complexes. These phosphorylation sites serve as binding sites for Pin1, which subsequently targets SMRT for degradation. Additionally, Pin1 may act in other pathways to affect tamoxifen resistance, as knockdown of Pin1 in BT-474 cells did not significantly up-regulate SMRT protein levels.