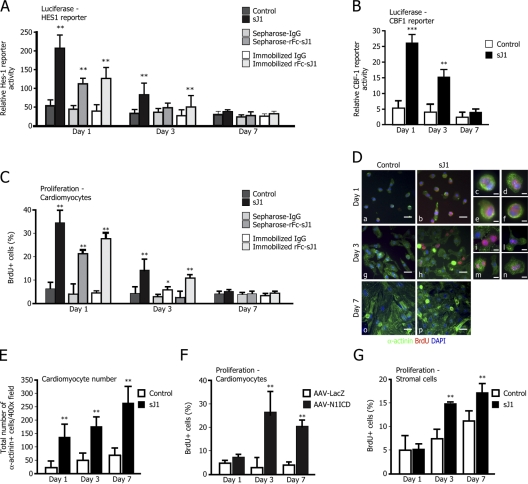
Figure 5.
sJ1 and Notch-ICD transduction enhance cardiomyocyte proliferation. (A) Treatment with sJ1 activates HES1 promoter transcription. Neonatal cardiomyocytes were assayed for HES1 reporter transactivation upon stimulation with conditioned medium from NIH-3T3 cells secreting sJ1 or rFc-sJ1 either preclustered on Sepharose beads or immobilized on plates. The respective controls were the supernatant from mock-transfected NIH-3T3 cells (Control), Sepharose-IgG, and immobilized IgG. The transactivation effect was evaluated at the indicated time points after reporter transfection. The histogram represents mean values ± SEM, n = 6. **, P < 0.001. (B) Treatment with sJ1 markedly enhances expression of the CBF1-luciferase reporter. Control or sJ1-conditioned medium (sJ1) was added to cardiomyocytes for 24 h after reporter transfection; the transactivation effect was evaluated at the indicated time points. The histogram represents mean values ± SEM, n = 6; **, P < 0.001. (C) sJ1 stimulation increases BrdU incorporation in cardiac myocytes. Neonatal cardiomyocytes were cultured in the presence of either sJ1- or control-conditioned medium, or either hIgG or rFc-sJ1 ligand preclustered onto Sepharose beads, and pulsed for BrdU incorporation. In a mirror experiment, neonatal cardiomyocytes were seeded onto rFc-sJ1 (or normal h-IgG as a control) precoated slides. After BrdU immunostaining, the total number of BrdU+ cells per well was scored. The histograms show mean ± SEM of the percentage of BrdU+ nuclei from at least three fields of ∼200 cells per field. Results are representative of at least three independent experiments. **, P < 0.001. (D) Low (panels a, b, g, h, o, and p)- and high (panels c–f and i–n)-magnification representative images of BrdU+ cardiomyocytes at different time points, after treatment with control- or sJ1-conditioned medium. Green, α-actinin; red, BrdU; blue, DNA (DAPI). Bars: (low magnification) 50 μm; (high magnification) 10 μm. The examples shown are representative of more than five experiments with similar outcomes. (E) sJ1 remarkably stimulates cardiomyocyte proliferation. Cardiac myocytes were cultured in the presence of sJ1 (sJ1)- or control-conditioned media. After specific immunostaining, the total number of α-actinin–positive cells per well was scored. The histogram shows mean ± SEM of at least 8 wells per time point from three independent experiments. **, P < 0.001. (F) AAV8-N1ICD strongly stimulates BrdU incorporation in cardiomyocytes. Neonatal cardiomyocytes were infected either with AAV8-LacZ or AAV8-N1ICD and pulsed for BrdU incorporation at different time points. After BrdU immunostaining, the total number of BrdU+ cells per well was scored. The histograms show mean ± SEM of the percentage of BrdU+ nuclei from at least three fields of ∼200 cells per field. Results are representative of at least three independent experiments. **, P < 0.001. (G) SJ1 stimulates BrdU incorporation in stromal cells. Stromal cells were cultured in the presence of sJ1 (sJ1)- or control-conditioned medium and pulsed for BrdU incorporation. After BrdU immunostaining, the total number of BrdU+ cells per well was scored. The histograms show mean ± SEM of the percentage of BrdU+ nuclei from at least three fields of ∼200 cells per field. **, P < 0.001.