Fig. 5. Asymmetric activation mechanism of a dimer of mGlu.
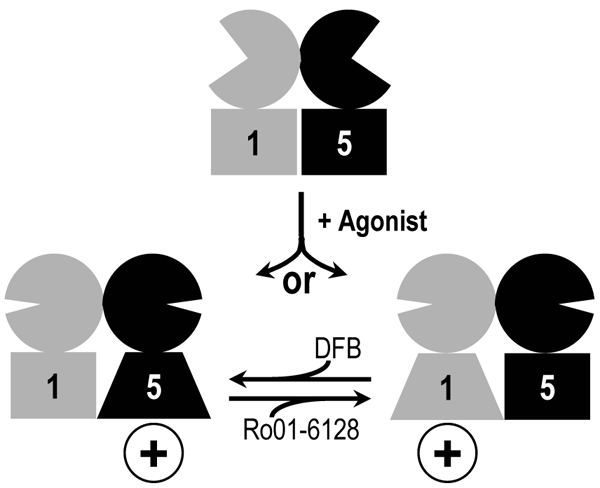
Schematic representation of the activation of a mGlu1:mGlu5 heterodimer and the effect of specific mGlu1 and mGlu5 positive allosteric modulators: Ro01-6128 and DFB, respectively. Agonist binding induces the closure of extracellular domains that triggers the activation of a single heptahelical domain or the other. The binding of a single positive allosteric modulator is sufficient to potentiate the dimer activity by further stabilizing the active state of the mGlu1 subunit (for Ro01-6128) or mGlu5 (for DFB).
