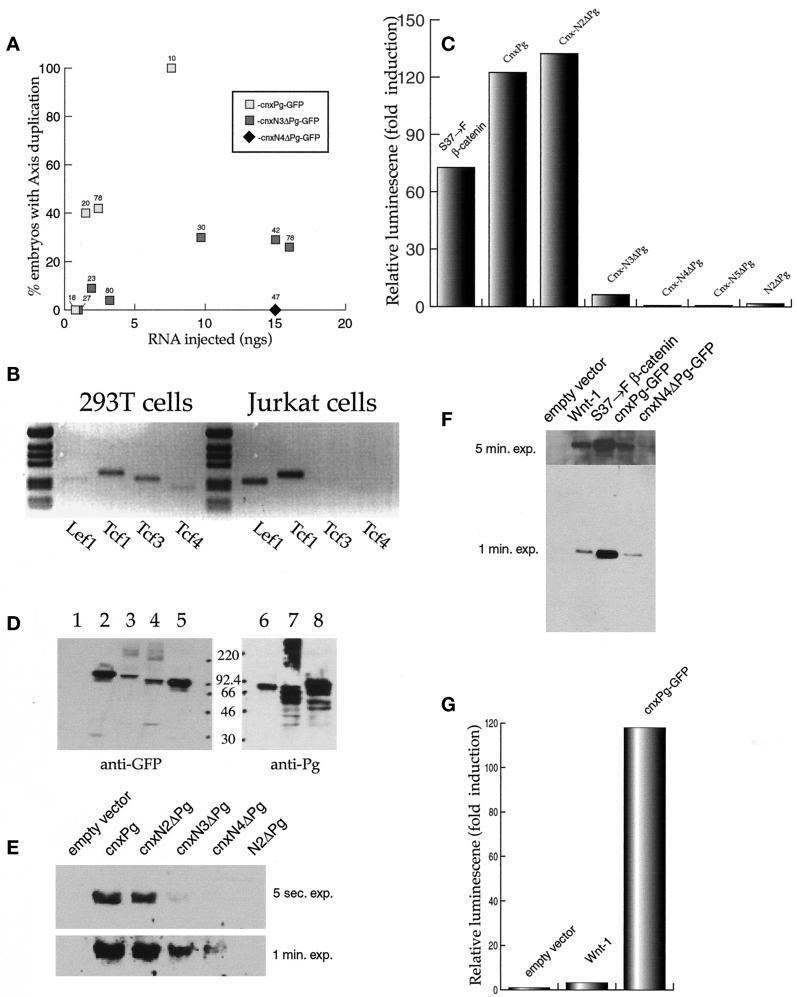
Figure 2.
The axis-inducing activities of the cnxPg-GFPs. (A) Fertilized Xenopus eggs, injected with various amounts of capped RNA (20 nl injected), were allowed to develop to stages 15–18 and then assayed for dorsal axis duplication. Only embryos that were fluorescent as a result of the expression of exogenous protein were counted; the numbers above each point indicate the total number of embryos scored. (B) Expression of TCFs in 293T cells. Reverse transcriptase-PCR analysis was used to analyze TCF expression. Human Jurkat cells express TCF1 and LEF1 RNAs. 293T cells express detectable levels of all four TCF RNAs, i.e., TCF1, LEF1, TCF3, and TCF4. Reactions in which ribonuclease was added showed no DNA amplification (our unpublished results). (C) cnxPg induction of the OT reporter. Human 293T cells were transfected with plasmids (2 μg of DNA) that express S37→F–β-catenin, N2ΔPg, or various forms of cnxPg, together with the OT reporter and a plasmid driving the expression of β-galactosidase. Luciferase activity was measured after 36 h and was normalized to β-galactosidase activity levels. All transfections used a total of 4.0 μg of DNA. Activity of the “empty vector” was normalized to 1. In each case, the pattern of reporter activation shown was reproduced in at least three independent experiments. (D) Accumulation of cnxPgs. 293T cells were transfected with 4 μg of plasmid DNA; 36 h later, the cells were homogenized and extracts were analyzed by immunoblot (lanes 1 and 6, empty vector; lane 2, cnxPg-GFP; lane 3, cnxN2ΔPg-GFP; lane 4, cnxN4ΔPg-GFP; lanes 5 and 8, cnxN5ΔPg-GFP; lane 7, cnxN3ΔPg) using either an anti-GFP antibody (lanes 1–5) or an anti-plakoglobin antibody (lanes 6–8). The cnxPg polypeptides consistently run with an apparent molecular weight lower than their calculated size. The positions of molecular weight markers are noted. (E) Stabilization of soluble β-catenin by cnxPgs. 293T cells were transfected with plasmids (2 μg of DNA) driving the expression of various cnxPgs or N2ΔPg; 36 h later, the cells were harvested, homogenized, and centrifuged to remove membrane-associated proteins, and soluble β-catenin was analyzed by immunoblot using an anti-β-catenin antibody. Two different exposures of the blot (5 s and 1 min) are shown. Very low levels of soluble β-catenin were seen in cultures transfected with the pCDNA3 plasmid (empty vector) alone. (F) A comparison of the stabilization of soluble β-catenin by Wnt-1 and cnxPg. 293T cells were cotransfected with plasmids that drove the expression of Wnt-1 (4 μg), S37→F–β-catenin (2 μg), cnxPg-GFP (2 μg), or cnxN4ΔPg-GFP (2 μg) and assayed 36 h later for soluble β-catenin, as in D (exposures of 1 and 5 min are shown). (G) The ability of cnxPg, Wnt-1, and S37→F–β-catenin plasmids to activate OT. 293T cells were transfected with cnxPgs or Wnt-1–expressing plasmids, and their ability to activate OT was measured. In a large number of experiments (>10), Wnt-1 produced only a modest increase in reporter activity, ranging from 2- to 10-fold.