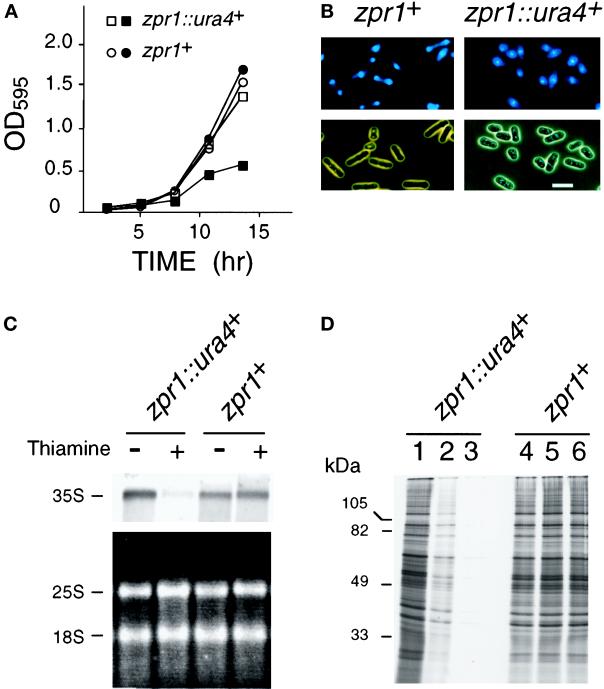
Figure 7.
Loss of zpr1+ function causes depletion of the rRNA precursor and decreased protein translation. (A) Wild-type haploid S. pombe (zpr1+) and the zpr1::ura4+ disrupted strain were transformed with the plasmid pREP41-zpr1 and grown in minimal liquid medium. The cultures were divided into two flasks in the absence (open symbols) and presence (closed symbols) of thiamine, respectively. Thiamine is a repressor of the nmt promoter located in the pREP plasmid. The growth of the cultures was monitored by measurement of the optical density at 595 nm. (B) The morphology of the yeast grown in the presence of thiamine (12 h) was examined by phase-contrast microscopy. DNA stained with 4,6-diamidino-2-phenylindole was visualized by epifluorescence. Bar, 10 μm. (C) Northern blot analysis of RNA isolated from the zpr1+ and the zpr1::ura4+ disrupted S. pombe strains transformed with the plasmid pREP41-zpr1. The yeast were grown in the absence and presence of the repressor thiamine (12 h). Ten micrograms of RNA isolated from these yeast were examined by denaturing agarose gel electrophoresis. The 25 and 18S mature rRNA were detected by staining with ethidium bromide (bottom panel). The 35S rRNA precursor was detected by Northern analysis using a 5′-ETS probe. An autoradiogram of the dried blot is shown (top panel). (D) Wild-type and zpr1::ura4+ disrupted strains were grown in the absence (lanes 1 and 4) and presence (lanes 2, 3, 5, and 6) of the repressor thiamine (12 h). The cells were diluted to the same density (0.2 OD595), labeled with [35S]methionine (150 μCi/ml) for 3 h, and harvested. The labeling with [35S]methionine was performed in the absence (lanes 1, 2, 4, and 5) and presence (lanes 3 and 6) of thiamine. Extracts prepared from the yeast were examined by SDS-PAGE and autoradiography.