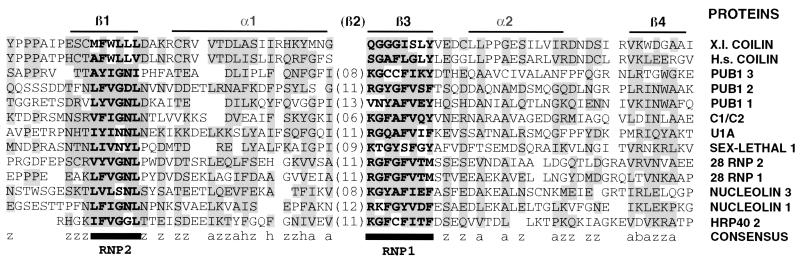
Figure 10.
The poly r(G)-binding region of human coilin compared with the corresponding region of Xenopus coilin (45% identity) and with the RNP consensus sequence from a selection of other proteins. Boxed amino acids at a given residue have similar properties: a, aromatic, h, hydrophobic, z, polar or charged, and b, basic. The RNP1 octamer and RNP2 hexamer are shown in bold. Putative α helices (α1, α2) and β sheets (β1, β2, β3) are based on structure determinations by Nagai et al. (1990) and Wittekind et al. (1992). The RNA-binding region of coilin is at best loosely related to the previously defined RNP consensus sequence.