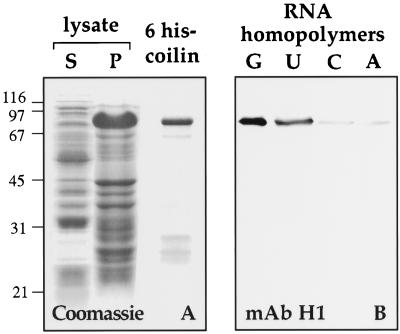
Figure 8.
Bacterially expressed Xenopus coilin binds to RNA homopolymers. (A) E. coli expressing 6 histidine-tagged Xenopus coilin were lysed and centrifuged to yield supernatant and pellet fractions. Proteins from these fractions were electrophoresed and stained with Coomassie blue (lanes S and P). The pellet fraction was solubilized with 8 M urea and bound to a Ni2+ column. Nearly pure 6 his-coilin was recovered from the column (6 his-coilin). (B) Purified 6 his-coilin was incubated with beads coated with RNA homopolymers. A Western blot of proteins that bound to the beads was probed with mAb H1. Purified 6 his-coilin bound to poly r(G) and poly r(U) but not to poly r(C) or poly r(A).