Abstract
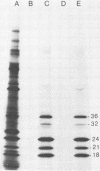
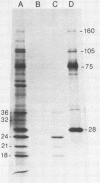
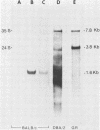
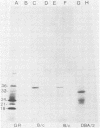
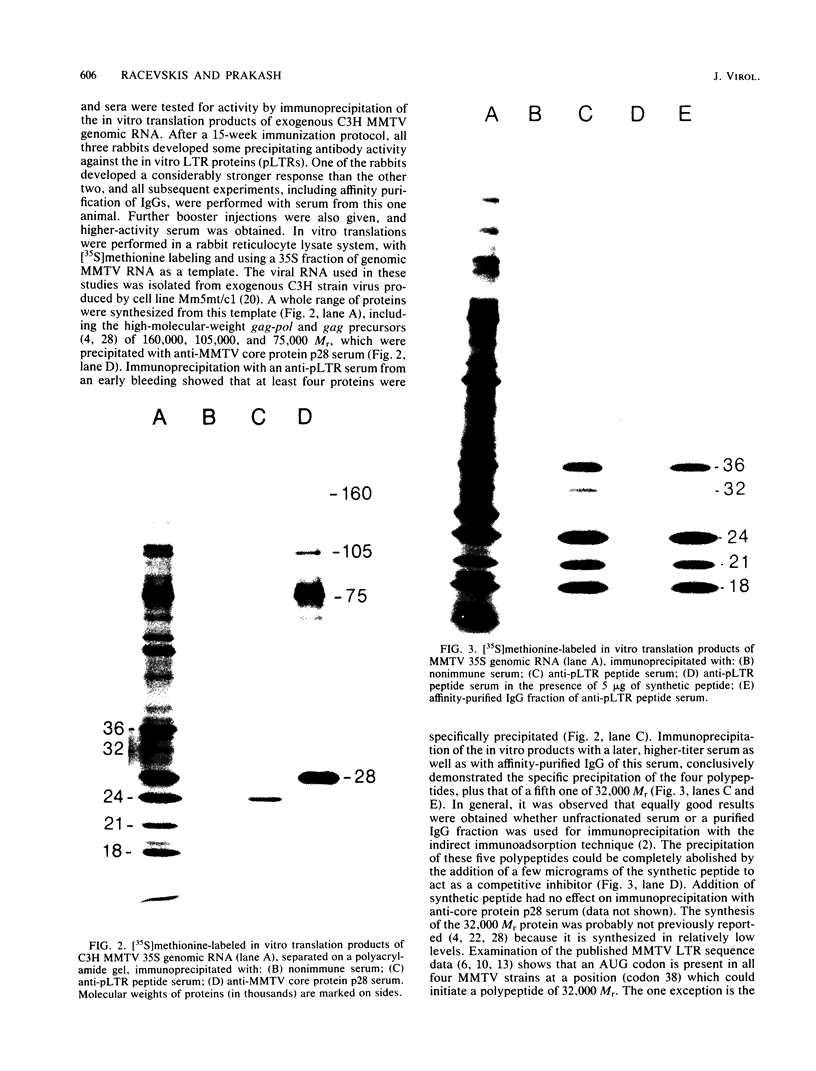
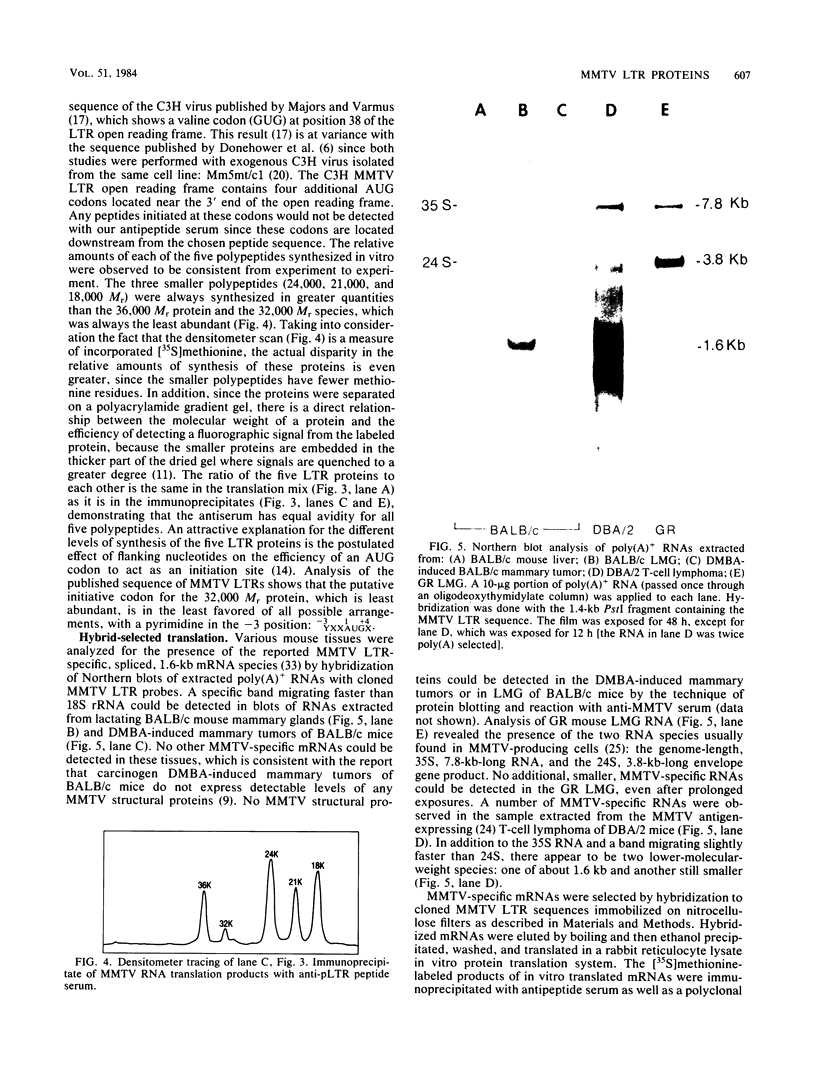
The long terminal repeat (LTR) region of mouse mammary tumor virus (MMTV) is known to contain an open reading frame of sufficient length to code for a protein of 36,000 Mr. The coding capacity of the 3' sequences of MMTV genomic RNA has been demonstrated by in vitro translation studies, which have reported the synthesis of four related proteins: p36, p24, p21, and p18. These proteins are overlapping translation products of the same open reading frame, with the smaller ones initiating at internal methionine codons. From the predicted amino acid sequence of the LTR protein, we have selected a region likely to be antigenic, obtained a synthetic peptide of that region, and raised antiserum to the peptide. The antipeptide serum specifically immunoprecipitated all four proteins from in vitro translated genomic 3' MMTV RNA, plus an additional one of 32,000 Mr. Published sequence data of MMRV LTRs show an internal AUG codon at a position which could initiate a protein of 32,000 Mr. The three smaller in vitro translation products (p24, p21, and p18) were consistently synthesized in much greater amounts than the p36 or p32 protein. The relative amount of each in vitro synthesized protein from genomic MMTV RNA could be predicted and was in good agreement with the postulated effect of flanking nucleotides on the efficiency of the respective AUG initiation codon. Polyadenylated RNAs, isolated from various mouse tissues, were selected by hybridization to plasmid DNA containing MMTV LTR sequences immobilized on nitrocellulose. In vitro translation of hybrid-selected mRNAs isolated from BALB/c mouse lactating mammary glands and carcinogen-induced mammary tumors, followed by immunoprecipitation with antipeptide serum, revealed that only one polypeptide was synthesized by the MMTV LTR-specific mRNA, the 36,000 Mr species.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bumol T. F., Reisfeld R. A. Unique glycoprotein-proteoglycan complex defined by monoclonal antibody on human melanoma cells. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1245–1249. doi: 10.1073/pnas.79.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. C., Majors J. E., Varmus H. E. Organization of mouse mammary tumor virus-specific DNA endogenous to BALB/c mice. J Virol. 1979 Nov;32(2):483–496. doi: 10.1128/jvi.32.2.483-496.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Peters G. Protein-coding potential of mouse mammary tumor virus genome RNA as examined by in vitro translation. J Virol. 1981 Jan;37(1):36–47. doi: 10.1128/jvi.37.1.36-47.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Smith R., Peters G. In vitro synthesis of polypeptides encoded by the long terminal repeat region of mouse mammary tumour virus DNA. Nature. 1981 Jun 11;291(5815):511–513. doi: 10.1038/291511a0. [DOI] [PubMed] [Google Scholar]
- Donehower L. A., Fleurdelys B., Hager G. L. Further evidence for the protein coding potential of the mouse mammary tumor virus long terminal repeat: nucleotide sequence of an endogenous proviral long terminal repeat. J Virol. 1983 Mar;45(3):941–949. doi: 10.1128/jvi.45.3.941-949.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower L. A., Huang A. L., Hager G. L. Regulatory and coding potential of the mouse mammary tumor virus long terminal redundancy. J Virol. 1981 Jan;37(1):226–238. doi: 10.1128/jvi.37.1.226-238.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley J. P., Rosen J. M., Butel J. S. Differential expression of poly(A)-adjacent sequences of mammary tumor virus RNA in murine mammary cells. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5797–5801. doi: 10.1073/pnas.75.12.5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusing-Swartz S., Medina D., Butel J. S., Socher S. H. Mouse mammary tumor virus genome expression in chemical carcinogen-induced mammary tumors in low- and high-tumor-incidence mouse strains. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5360–5364. doi: 10.1073/pnas.76.10.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasel N., Pearson K., Buetti E., Diggelmann H. The region of mouse mammary tumor virus DNA containing the long terminal repeat includes a long coding sequence and signals for hormonally regulated transcription. EMBO J. 1982;1(1):3–7. doi: 10.1002/j.1460-2075.1982.tb01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C. R., Scott I. R. Fluorography--limitations on its use for the quantitative detection of 3H- and 14C-labeled proteins in polyacrylamide gels. Anal Biochem. 1983 Mar;129(2):371–376. doi: 10.1016/0003-2697(83)90564-x. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy N., Knedlitschek G., Groner B., Hynes N. E., Herrlich P., Michalides R., van Ooyen A. J. Long terminal repeats of endogenous mouse mammary tumour virus contain a long open reading frame which extends into adjacent sequences. Nature. 1982 Feb 18;295(5850):622–624. doi: 10.1038/295622a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner R. A., Green N., Alexander H., Liu F. T., Sutcliffe J. G., Shinnick T. M. Chemically synthesized peptides predicted from the nucleotide sequence of the hepatitis B virus genome elicit antibodies reactive with the native envelope protein of Dane particles. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3403–3407. doi: 10.1073/pnas.78.6.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majors J. E., Varmus H. E. Nucleotide sequences at host-proviral junctions for mouse mammary tumour virus. Nature. 1981 Jan 22;289(5795):253–258. doi: 10.1038/289253a0. [DOI] [PubMed] [Google Scholar]
- Majors J. E., Varmus H. E. Nucleotide sequencing of an apparent proviral copy of env mRNA defines determinants of expression of the mouse mammary tumor virus env gene. J Virol. 1983 Sep;47(3):495–504. doi: 10.1128/jvi.47.3.495-504.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A., Walter G., Singer S. J. On the nature of crossreactions observed with antibodies directed to defined epitopes. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5939–5943. doi: 10.1073/pnas.79.19.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens R. B., Hackett A. J. Tissue culture studies of mouse mammary tumor cells and associated viruses. J Natl Cancer Inst. 1972 Nov;49(5):1321–1332. [PubMed] [Google Scholar]
- Pachl C., Biegalke B., Linial M. RNA and protein encoded by MH2 virus: evidence for subgenomic expression of v-myc. J Virol. 1983 Jan;45(1):133–139. doi: 10.1128/jvi.45.1.133-139.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Smith R., Brookes S., Dickson C. Conservation of protein coding potential in the long terminal repeats of exogenous and endogenous mouse mammary tumor viruses. J Virol. 1982 Jun;42(3):880–888. doi: 10.1128/jvi.42.3.880-888.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash O., Guntaka R. V., Sarkar N. H. Evidence for a prokaryotic promoter in the murine mammary tumor virus long terminal repeat. Gene. 1983 Aug;23(2):117–130. doi: 10.1016/0378-1119(83)90043-4. [DOI] [PubMed] [Google Scholar]
- Racevskis J., Sarkar N. H. ML antigen of DBA/2 mouse leukemias: expression of an endogenous murine mammary tumor virus. J Virol. 1982 Jun;42(3):804–813. doi: 10.1128/jvi.42.3.804-813.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. L., Varmus H. E. Dexamethasone induction of the intracellular RNAs of mouse mammary tumor virus. J Virol. 1981 Dec;40(3):673–682. doi: 10.1128/jvi.40.3.673-682.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. M., Rabin E. H., Oroszlan S. Post-translational modification of Rauscher leukemia virus precursor polyproteins encoded by the gag gene. J Virol. 1979 Apr;30(1):255–266. doi: 10.1128/jvi.30.1.255-266.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwinghamer M. W., Shepherd R. J. Formaldehyde-containing slab gels for analysis of denatured, tritium-labeled RNA. Anal Biochem. 1980 Apr;103(2):426–434. doi: 10.1016/0003-2697(80)90634-x. [DOI] [PubMed] [Google Scholar]
- Sen G. C., Racevskis J., Sarkar N. H. Synthesis of murine mammary tumor viral proteins in vitro. J Virol. 1981 Mar;37(3):963–975. doi: 10.1128/jvi.37.3.963-975.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telang N. T., Banerjee M. R., Iyer A. P., Kundu A. B. Neoplastic transformation of epithelial cells in whole mammary gland in vitro. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5886–5890. doi: 10.1073/pnas.76.11.5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M. Structure, variation and synthesis of retrovirus long terminal repeat. Cell. 1981 Nov;27(1 Pt 2):1–3. doi: 10.1016/0092-8674(81)90353-6. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Walter G., Hutchinson M. A., Hunter T., Eckhart W. Antibodies specific for the polyoma virus middle-size tumor antigen. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4882–4886. doi: 10.1073/pnas.78.8.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler D. A., Butel J. S., Medina D., Cardiff R. D., Hager G. L. Transcription of mouse mammary tumor virus: identification of a candidate mRNA for the long terminal repeat gene product. J Virol. 1983 Apr;46(1):42–49. doi: 10.1128/jvi.46.1.42-49.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Enami J., Nandi S. Regulation of mammary tumor virus production by prolactin in BALB/cfC3H mouse normal mammary epithelial cells in vitro. Cancer Res. 1977 Oct;37(10):3644–3647. [PubMed] [Google Scholar]