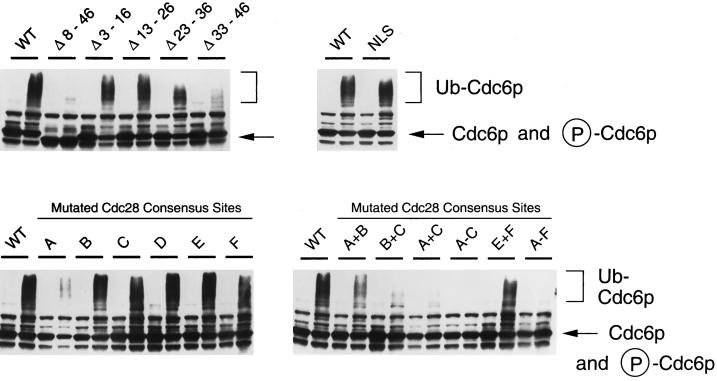
Figure 6.
In vitro ubiquitination of Cdc6p mutants. Wild-type and mutant Cdc6-Myc9p were produced by in vitro translation as described in MATERIALS AND METHODS, and ubiquitination reactions were carried out as described. For each pair of samples, the reaction in the right lane was a complete ubiquitination reaction (DEAE-fractionated extract of G1 arrested RJD885 cells, partially purified Cdc34p, insect cell lysate containing Clb5p/Cdc28p kinase, an ATP-regenerating system, salts, and ubiquitin). For the reactions run in the left lane, Cdc34p was omitted. After the ubiquitination reaction, samples were treated with RNase (as in Figure 4), mixed with SDS-PAGE loading buffer, and subjected to electrophoreses on 7.5% gels. Proteins were blotted to Hybond nitrocellulose and probed with 9E10 antibody, and the Western blots were developed with the Super Signal luminescent reagent (Pierce).