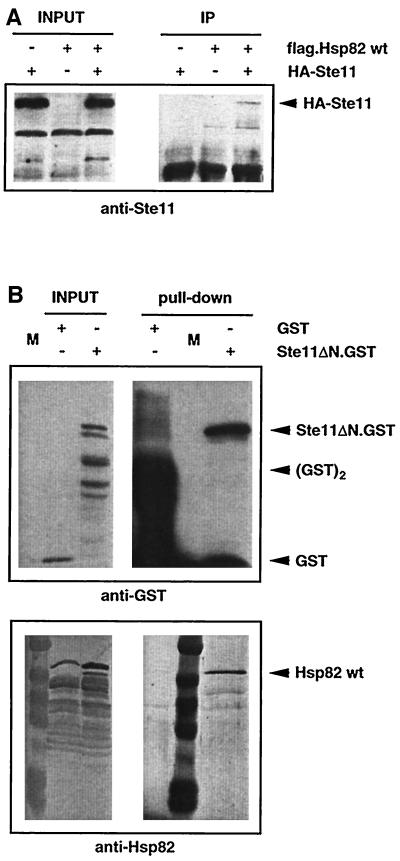
Figure 5.
Ste11 forms complexes with Hsp90. (A) Immunoprecipitation experiment showing association of HA-tagged Ste11 with FLAG-tagged Hsp82. INPUT and IP designate equal amounts of total extract and immunoprecipitates, respectively (exposure times are not the same). Note that isogenic strains were used expressing Hsp82 with or without the FLAG tag. HA-Ste11 was revealed by immunoblotting with an anti-Ste11 antiserum. (B) GST pull-down experiment showing association of Ste11ΔN.GST with endogenous Hsp90 (Hsp82/Hsc82). INPUT and “Pull-down” designate equal amounts of total extract and proteins purified with glutathione-sepharose beads, respectively (exposure times are not the same). GST and Ste11ΔN.GST and Hsp82/Hsc82 were revealed by probing the same blot with anti-GST and chicken anti-Hsp82 antibodies, respectively. Note that unfused GST binds the affinity matrix more efficiently, resulting in very strong GST monomer and dimer bands.