Abstract
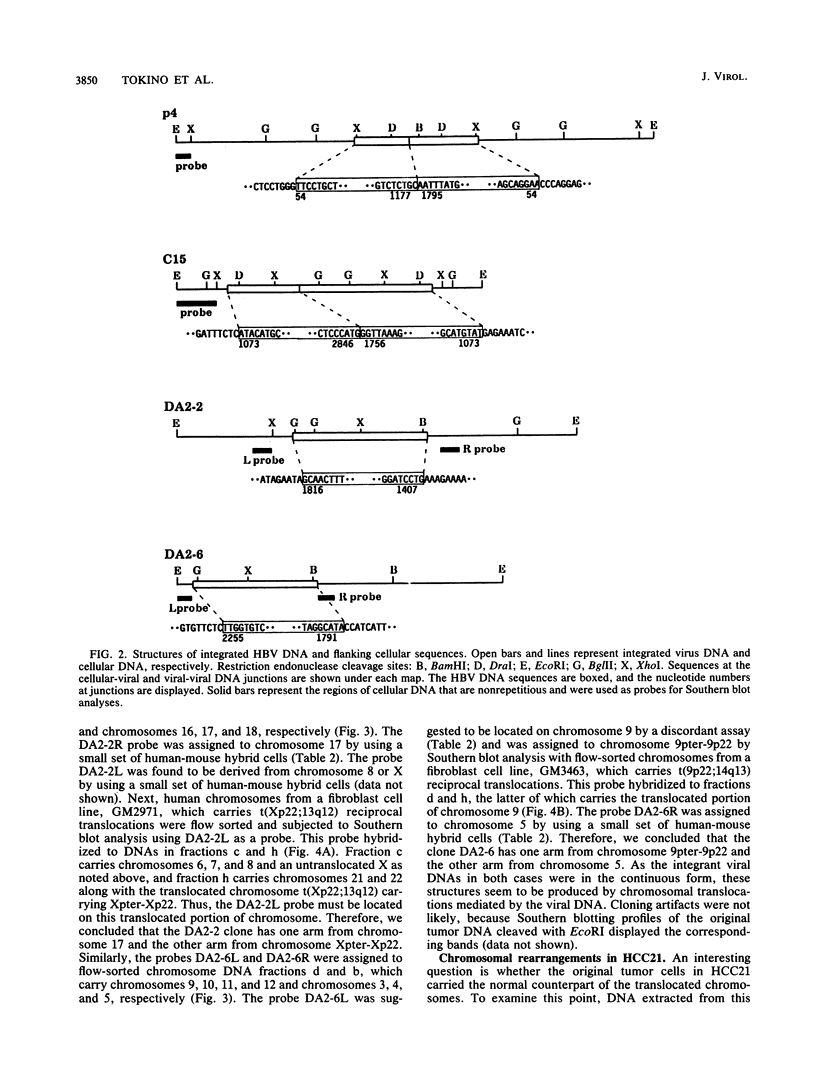
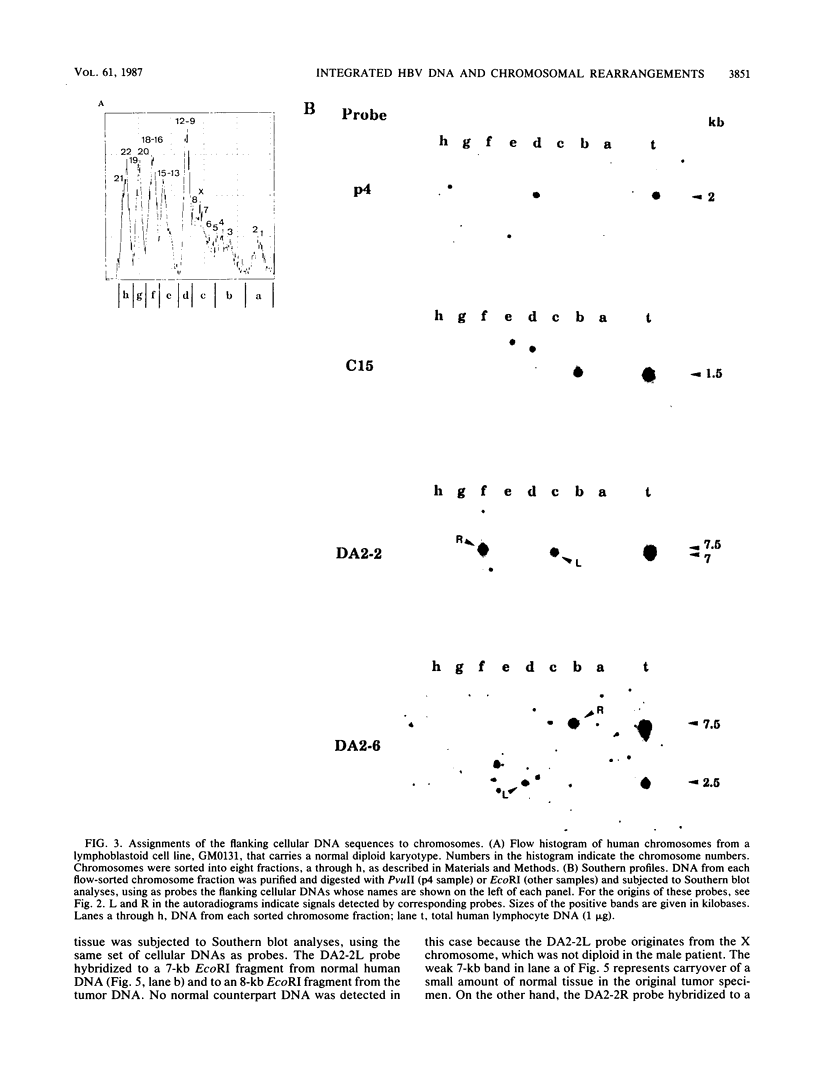
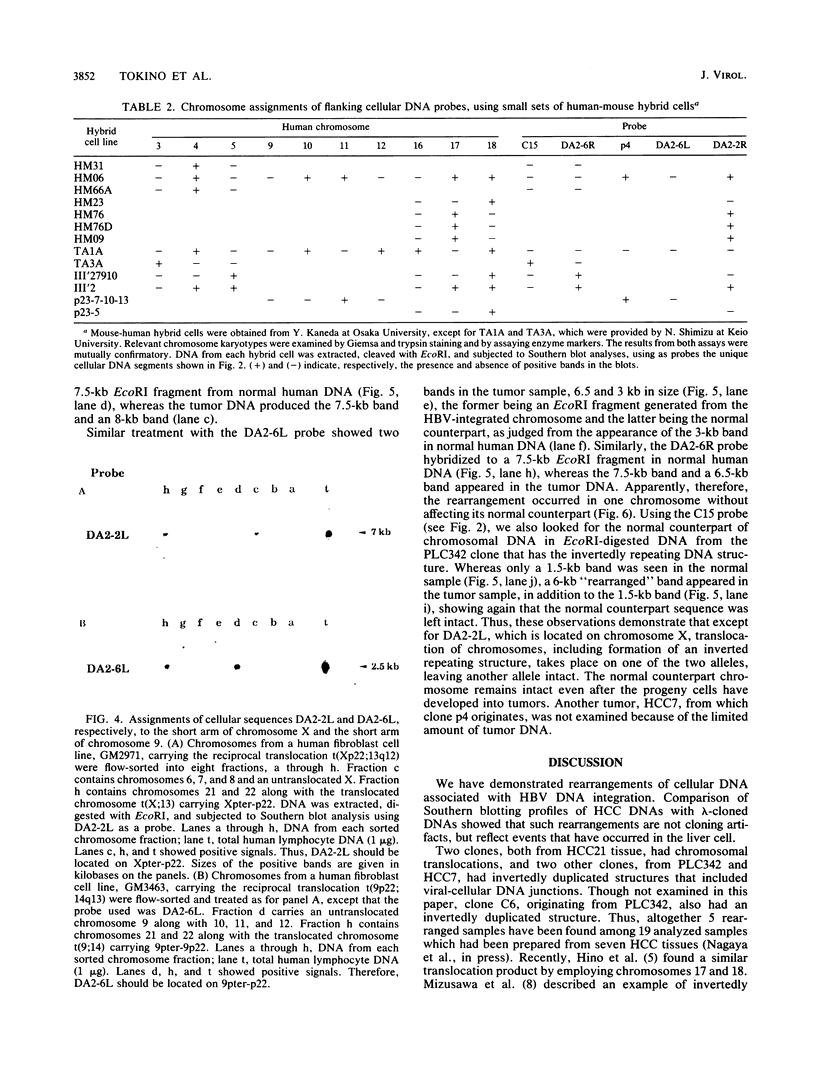
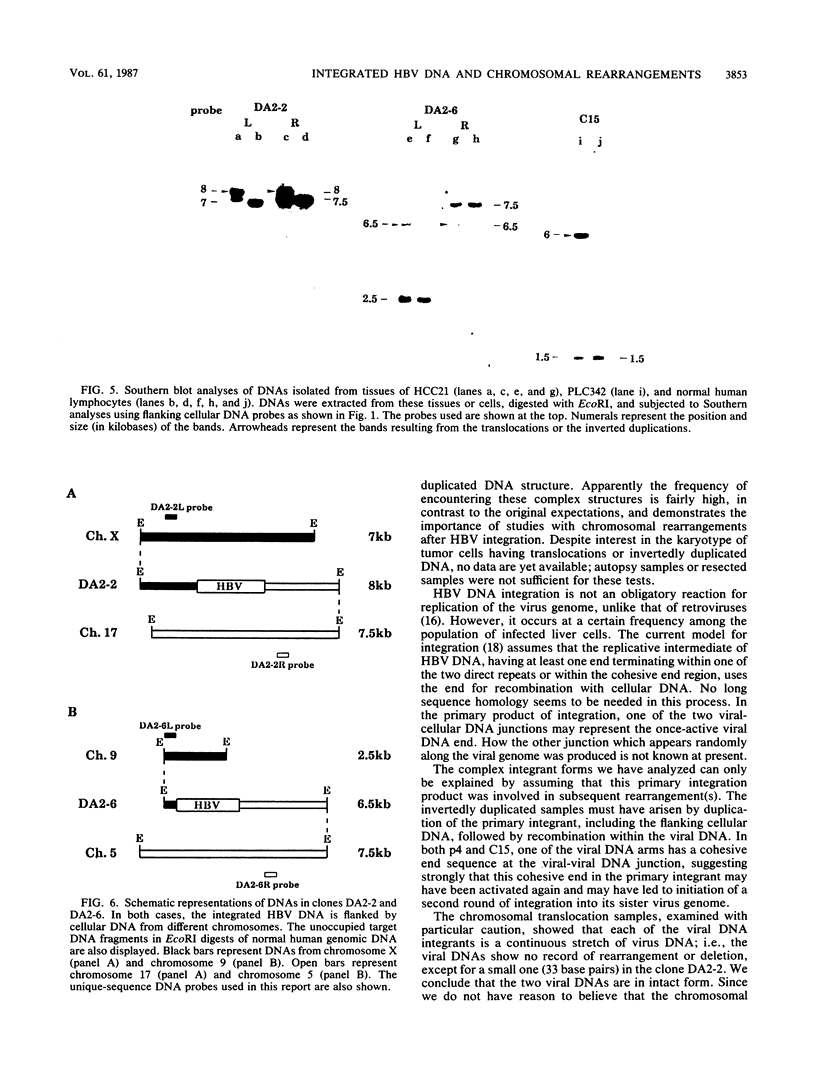
Integrated hepatitis B virus (HBV) DNA is found in hepatocellular carcinomas which develop in HBV carriers. Presented here are the results of analyses of four integrants that show chromosomal rearrangements associated with the integrated HBV DNA. Two clones (p4 and C15) were found to have large inverted repeating structures, each consisting of HBV genome along with flanking cellular sequences. The structure must have arisen by duplication of the primary integrant, including the flanking cellular DNA, followed by recombination within the viral DNA. One of the two viral arms in each clone joins to the other viral arm at the "cohesive end region." Two clones (DA2-2 and DA2-6) were found to have integrated HBV sequences, each flanked by cellular DNAs from different chromosomes (chromosome X joined to 17 and chromosome 5 joined to 9). They must be the products of cellular DNA translocations using the integrated HBV DNA as the switch point. The viral DNA in each clone is a continuous stretch of a single virus genome with one end in the cohesive end region. These complex structures seem to have been produced by activation of the cohesive end of an integrant viral genome, followed by its recombination with another chromosomal DNA.
Full text
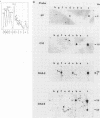
PDF
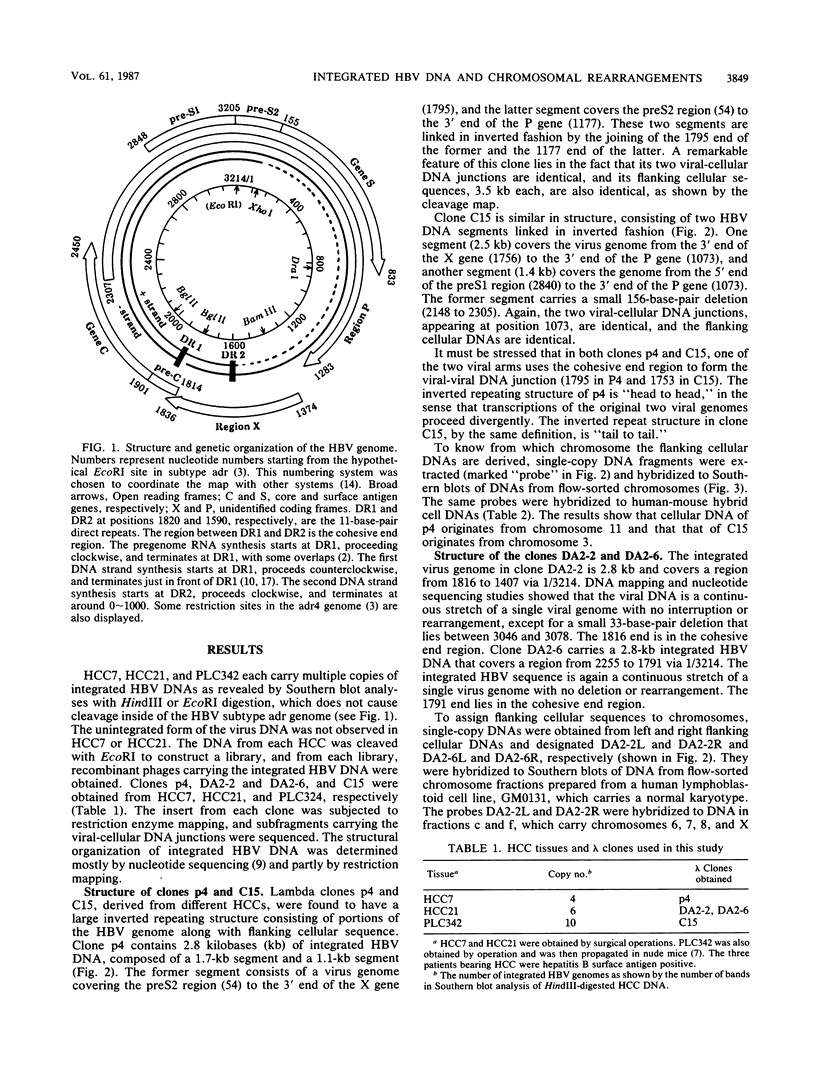

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dejean A., Sonigo P., Wain-Hobson S., Tiollais P. Specific hepatitis B virus integration in hepatocellular carcinoma DNA through a viral 11-base-pair direct repeat. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5350–5354. doi: 10.1073/pnas.81.17.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders G. H., Ganem D., Varmus H. Mapping the major transcripts of ground squirrel hepatitis virus: the presumptive template for reverse transcriptase is terminally redundant. Cell. 1985 Aug;42(1):297–308. doi: 10.1016/s0092-8674(85)80125-2. [DOI] [PubMed] [Google Scholar]
- Fujiyama A., Miyanohara A., Nozaki C., Yoneyama T., Ohtomo N., Matsubara K. Cloning and structural analyses of hepatitis B virus DNAs, subtype adr. Nucleic Acids Res. 1983 Jul 11;11(13):4601–4610. doi: 10.1093/nar/11.13.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushige S., Murotsu T., Matsubara K. Chromosomal assignment of human genes for gastrin, thyrotropin (TSH)-beta subunit and C-erbB-2 by chromosome sorting combined with velocity sedimentation and Southern hybridization. Biochem Biophys Res Commun. 1986 Jan 29;134(2):477–483. doi: 10.1016/s0006-291x(86)80445-4. [DOI] [PubMed] [Google Scholar]
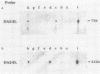
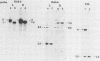
- Hino O., Shows T. B., Rogler C. E. Hepatitis B virus integration site in hepatocellular carcinoma at chromosome 17;18 translocation. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8338–8342. doi: 10.1073/pnas.83.21.8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S., Freytag von Loringhoven A., Kahmann R., Hofschneider P. H., Koshy R. The genetic organization of integrated hepatitis B virus DNA in the human hepatoma cell line PLC/PRF/5. Nucleic Acids Res. 1984 Sep 11;12(17):6871–6886. doi: 10.1093/nar/12.17.6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T., Takano M., Miyamoto K., Itoh Y., Yoshizawa H., Koike M., Mochizuki T., Tanaka E., Okamoto H., Imai M. Nude mice bearing human primary hepatocellular carcinoma that produces hepatitis B surface, core, and e antigens, as well as deoxyribonucleic acid polymerase. Gastroenterology. 1986 Jan;90(1):135–142. doi: 10.1016/0016-5085(86)90085-5. [DOI] [PubMed] [Google Scholar]
- Mizusawa H., Taira M., Yaginuma K., Kobayashi M., Yoshida E., Koike K. Inversely repeating integrated hepatitis B virus DNA and cellular flanking sequences in the human hepatoma-derived cell line huSP. Proc Natl Acad Sci U S A. 1985 Jan;82(1):208–212. doi: 10.1073/pnas.82.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger C., Ganem D., Varmus H. E. Biochemical and genetic evidence for the hepatitis B virus replication strategy. Science. 1986 Apr 25;232(4749):477–484. doi: 10.1126/science.3961490. [DOI] [PubMed] [Google Scholar]
- Sillar R., Young B. D. A new method for the preparation of metaphase chromosomes for flow analysis. J Histochem Cytochem. 1981 Jan;29(1):74–78. doi: 10.1177/29.1.6162882. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Summers J., Mason W. S. Replication of the genome of a hepatitis B--like virus by reverse transcription of an RNA intermediate. Cell. 1982 Jun;29(2):403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- Tsurimoto T., Fujiyama A., Matsubara K. Stable expression and replication of hepatitis B virus genome in an integrated state in a human hepatoma cell line transfected with the cloned viral DNA. Proc Natl Acad Sci U S A. 1987 Jan;84(2):444–448. doi: 10.1073/pnas.84.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will H., Reiser W., Weimer T., Pfaff E., Büscher M., Sprengel R., Cattaneo R., Schaller H. Replication strategy of human hepatitis B virus. J Virol. 1987 Mar;61(3):904–911. doi: 10.1128/jvi.61.3.904-911.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaginuma K., Kobayashi M., Yoshida E., Koike K. Hepatitis B virus integration in hepatocellular carcinoma DNA: duplication of cellular flanking sequences at the integration site. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4458–4462. doi: 10.1073/pnas.82.13.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemer M., Garcia P., Shaul Y., Rutter W. J. Sequence of hepatitis B virus DNA incorporated into the genome of a human hepatoma cell line. J Virol. 1985 Mar;53(3):885–892. doi: 10.1128/jvi.53.3.885-892.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]