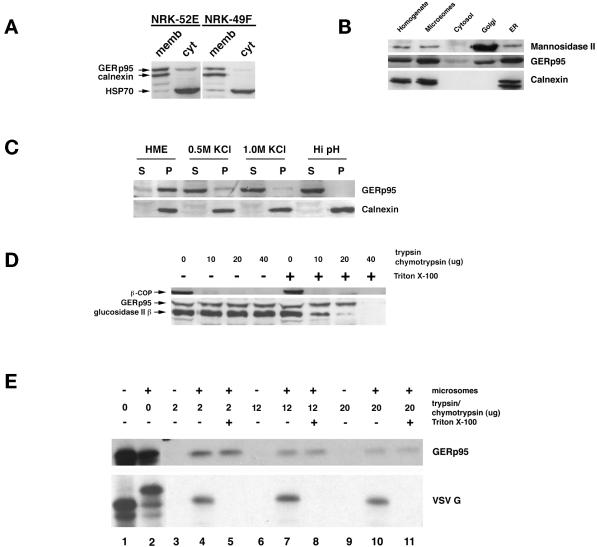
Figure 7.
Interaction of GERp95 with membranes in vivo and in vitro. (A) Crude microsomes and cytosol were prepared from NRK52E and NRK49F cells, and equivalent proportions normalized to starting volumes were separated by SDS-PAGE and transferred to PVDF membranes. The membranes were probed with rabbit antisera to GERp95, calnexin, and rat anti-HSP70. The majority of GERp95 and calnexin are found in the membrane fractions, whereas HSP70 is in the soluble fraction. (B) Fifty micrograms (protein) of rat liver fractions were subjected to SDS-PAGE and immunoblotting with antibodies to GERp95, and calnexin (ER marker) and Man II (Golgi). The highest concentrations of GERp95 are found in rough ER fractions. (C) NRK52E microsomes were extracted with HME, 0.5 M KCl, 1.0 M KCl, or Hi pH buffer (0.1 M sodium carbonate, pH 11.5), and then centrifuged at 100,000 × g for 60 min to obtain pellet (P) and soluble (S) fractions. Alkaline treatment and KCl washing resulted in complete extraction of GERp95 from membranes. (D) Microsomes were incubated at 0°C with increasing amounts of trypsin/chymotrypsin with or without 1% Triton X-100 followed by SDS-PAGE and immunoblotting with rabbit antiserum to GERp95, β-COP, and glucosidase II β-subunit. In the absence of detergent, GERp95 and glucosidase II β are insensitive to protease, but are completely digested when Triton X-100 is included. In contrast, β-COP, a peripheral membrane protein on the cytosolic side of membranes is completely degraded by the proteases even in the absence of detergent. (E) 35S-labeled GERp95 was synthesized in vitro in the presence or absence of canine pancreatic microsomes. Samples were subjected to digestion with varying amounts of trypsin/chymotrypsin in the presence or absence of Triton X-100 before SDS-PAGE and autoradiography. VSV G protein was used as a positive control to show translocational activity of the microsomes. VSV G is only protected from protease when microsomes are present and detergent is absent.