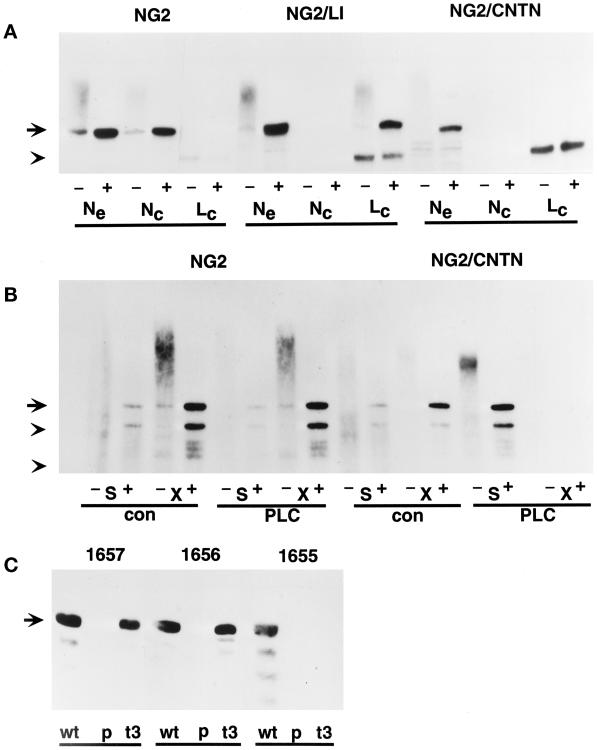
Figure 2.
Characterization of transfected molecules. U251 cells transfected with wild-type NG2 (U251NG2.51), the NG2/CNTN chimera (U251NG2/CNTN.40), the NG2/L1 chimera (U251NG2/L1.7), and the truncated NG2/t3 mutant (U251NG2/t3.4) were characterized by the following immunochemical criteria. (A) NP40 extracts of 125I-labeled U251NG2.51, U251NG2/CNTN.40, and U251NG2/L1.7 cells were used for immunoprecipitation studies with the rabbit antibodies NG2/EC (Ne), 1466 (Nc), and 1465 (Lc). The locations of the epitopes recognized by these antisera are shown in Figure 1. Half of each immunoprecipitate was treated with chondroitinase ABC to remove chondroitin sulfate (+), whereas the other half was left untreated (−). Samples were subjected to SDS-PAGE analysis on 3–20% gradient gels. The position of the 300-kDa NG2 core protein is marked in the left margin by an arrow. The arrowhead marks the position of a nonspecifically precipitated 200-kDa component. Molecules containing the NG2 ectodomain are precipitated by NG2/EC in all three cases. In contrast, the 1466 antibody precipitates only the wild-type NG2, which contains the NG2 cytoplasmic domain, and 1465 precipitates only the NG2/L1 chimera, which contains the L1 cytoplasmic domain. (B) 125I-labeled U251NG2.51 (NG2) and U251NG2/CNTN.40 (NG2/CNTN) cells were suspended in 0.2 ml of PBS and divided into two aliquots. One aliquot was treated for 10 min at 37°C with 5 U/ml PI-PLC (PLC), and the other was incubated under similar conditions without the enzyme (con). After these incubations, centrifugation was used to separate supernatants and cell pellets. Both the supernatants (S) and NP40 extracts of the cell pellets (X) were used for immunoprecipitation with the NG2/EC antibody. Half of each immunoprecipitate was treated with chondroitinase ABC (+), and the other half was left untreated (−). Samples were then analyzed by SDS-PAGE on 3–20% gradient gels. The autoradiograms of these gels show that PI-PLC treatment of the U251NG2/CNTN.40 cells causes quantitative release of the NG2/CNTN chimera into the supernatant. In contrast, wild-type NG2 remains associated with the cell surface during PI-PLC treatment. The arrow at left indicates the position of the 300-kDa NG2 core protein. Arrowheads mark the positions of 200- and 116-kDa molecular mass standards. (C) NP40 extracts of U251NG2.51 (wt), U251NG2/t3.4 (t3), and parental U251 cells (p) were treated with chondroitinase ABC, electrophoresed on 3–20% SDS-PAGE gels, and electrophoretically transferred to Immobilon P membranes. After blocking, the membranes were probed with rabbit antibodies specific for defined segments of the membrane-proximal NG2 ectodomain (1657) and cytoplasmic domain (1655 and 1656; see Figure 1). Both wild-type NG2 and the NG2/t3 mutant are recognized by the 1657 and 1656 antisera, but only wild-type NG2 is recognized by the C-terminal 1655 antibody. Parental cells do not contain immunoreactive NG2 species. The arrow at left indicates the position of the 300-kDa NG2 core protein.