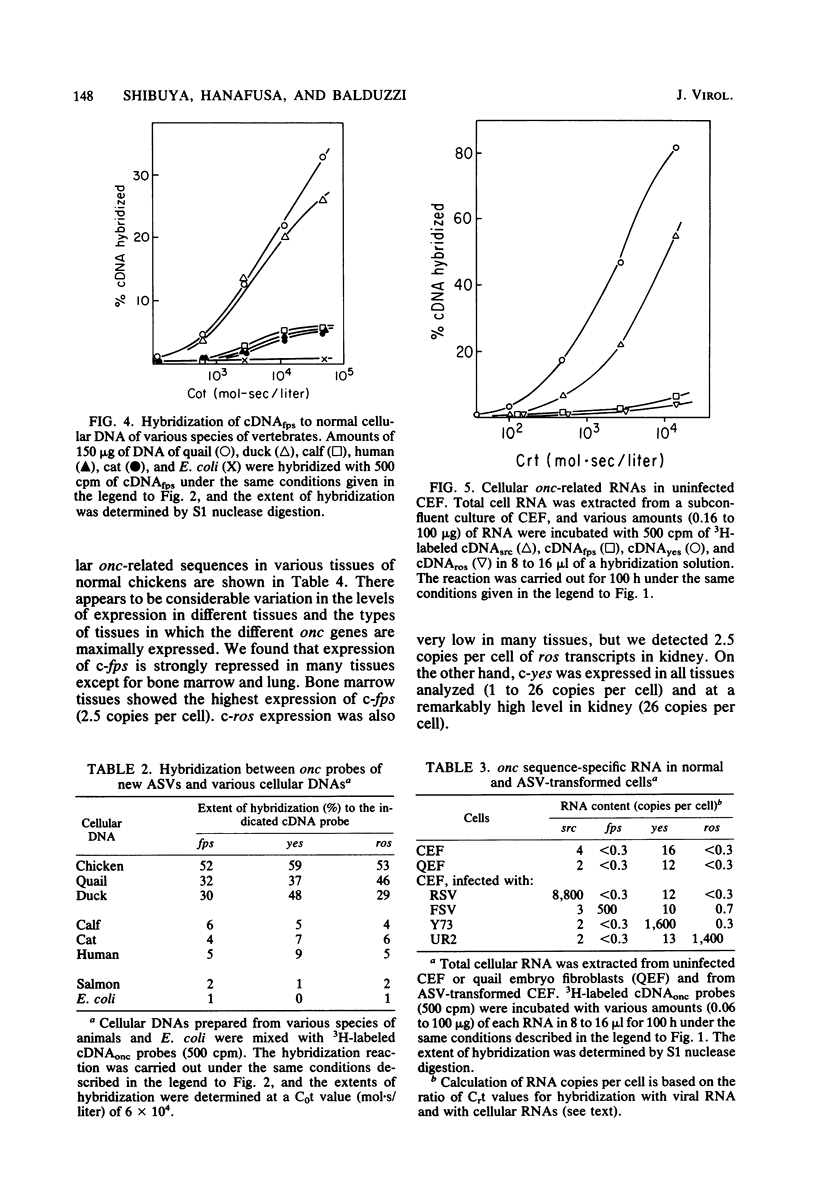
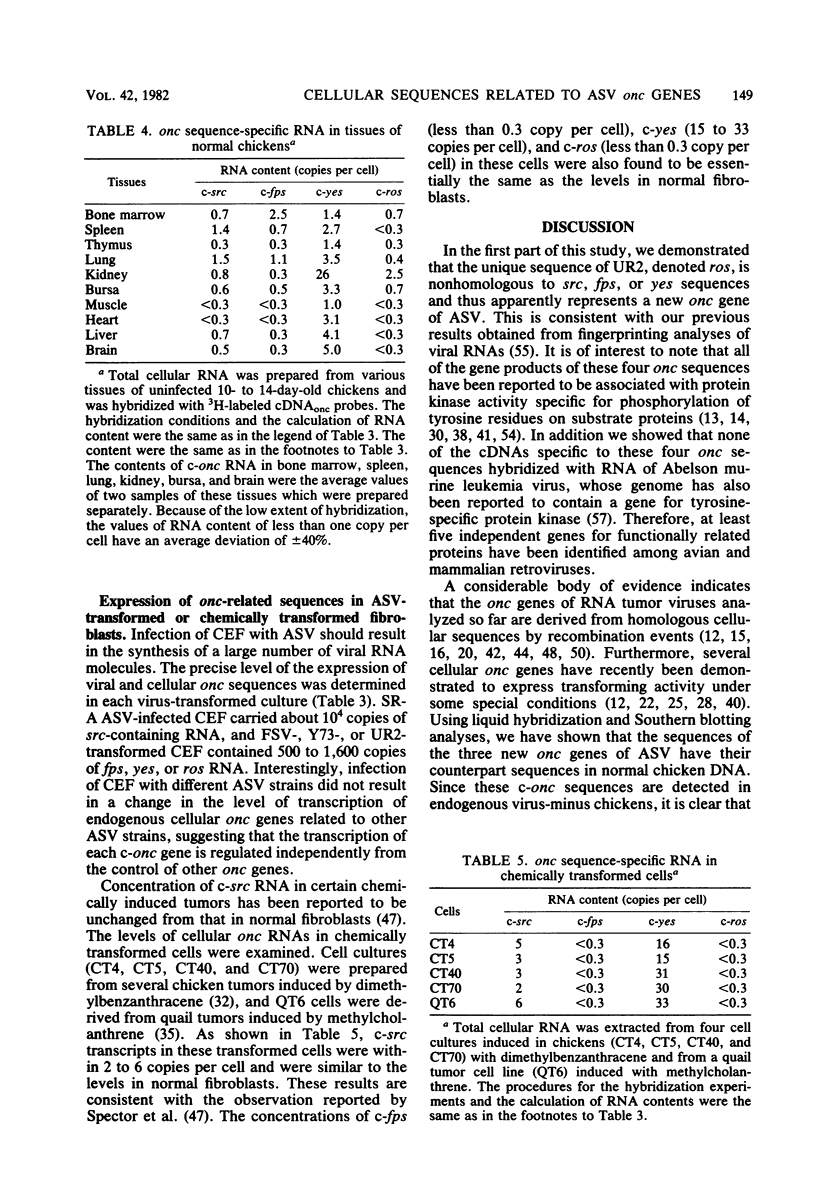
Abstract
Two onc genes of avian sarcoma viruses unrelated to the src gene have recently been identified: fps of Fujinami sarcoma virus/PRCII/UR1 and yes of Y73/Esh sarcoma virus. In the first part of this study we demonstrated that UR2, the most recently isolated avian sarcoma virus, contains in its genome a unique sequence, ros, nonhomologous to src, fps, and yes sequences or to transforming genes of avian acute leukemia viruses. Using cDNAs specific to the inserts of avian sarcoma virus genomes, we examined the existence and the transcription of cellular nucleotide sequences related to the three new onc genes of avian sarcoma virus (fps, yes and ros) in various cells. The progenitor cellular sequences for these onc genes (c-onc) were present in uninfected chicken DNA in one or few copies per haploid genome. These c-onc sequences were detectable in cellular DNA of a wide variety of vertebrates, and the homology between viral and cellular onc was inversely related to the phylogenetic distance of animal species. The pattern of expression of these c-onc genes in different tissues of chickens was found to be unique to each gene. The expression of c-fps and c-ros genes was generally repressed in many tissues, but c-fps was expressed at higher levels in bone marrow (2.5 copies per cell) and lung (1.1 copies per cell), whereas c-ros was mainly transcribed in kidney (2.5 copies per cell). On the other hand, c-yes transcripts were easily detectable in all tissues analyzed and were found at high levels in kidney (26 copies per cell). These c-onc expressions were unaffected by infection with avian sarcoma viruses that contained other onc genes. In a few cultures of chicken and quail transformed cells derived from tumors induced by chemical carcinogens, we found that the levels of transcription of the four c-onc genes remained unaltered, compared with that in normal tissues.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson H. T., Rabstein L. S. Lymphosarcoma: virus-induced thymic-independent disease in mice. Cancer Res. 1970 Aug;30(8):2213–2222. [PubMed] [Google Scholar]
- Astrin S. M., Buss E. G., Haywards W. S. Endogenous viral genes are non-essential in the chicken. Nature. 1979 Nov 15;282(5736):339–341. doi: 10.1038/282339a0. [DOI] [PubMed] [Google Scholar]
- Balduzzi P. C., Notter M. F., Morgan H. R., Shibuya M. Some biological properties of two new avian sarcoma viruses. J Virol. 1981 Oct;40(1):268–275. doi: 10.1128/jvi.40.1.268-275.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M., Beemon K., Devare S. G. Origin and functional properties of the major gene product of the Snyder-Theilen strain of feline sarcoma virus. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5158–5162. doi: 10.1073/pnas.77.9.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M., Courtneidge S. A., Levinson A. D., Oppermann H., Quintrell N., Sheiness D. K., Weiss S. R., Varmus H. E. Origin and function of avian retrovirus transforming genes. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):919–930. doi: 10.1101/sqb.1980.044.01.099. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- CARR J. G., CAMPBELL J. G. Three new virus-induced fowl sarcomata. Br J Cancer. 1958 Dec;12(4):631–635. doi: 10.1038/bjc.1958.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H. Expression of endogenous avian myeloblastosis virus information in different chicken cells. J Virol. 1980 Oct;36(1):162–170. doi: 10.1128/jvi.36.1.162-170.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Purchio A. F., Brugge J. S., Erikson R. L. A normal cell protein similar in structure and function to the avian sarcoma virus transforming gene product. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3159–3163. doi: 10.1073/pnas.76.7.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFeo D., Gonda M. A., Young H. A., Chang E. H., Lowy D. R., Scolnick E. M., Ellis R. W. Analysis of two divergent rat genomic clones homologous to the transforming gene of Harvey murine sarcoma virus. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3328–3332. doi: 10.1073/pnas.78.6.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. A., Hanafusa T., Hanafusa H. Characterization of protein kinase activity associated with the transforming gene product of Fujinami sarcoma virus. Cell. 1980 Dec;22(3):757–765. doi: 10.1016/0092-8674(80)90552-8. [DOI] [PubMed] [Google Scholar]
- Feldman R. A., Wang L. H., Hanafusa H., Balduzzi P. C. Avian sarcoma virus UR2 encodes a transforming protein which is associated with a unique protein kinase activity. J Virol. 1982 Apr;42(1):228–236. doi: 10.1128/jvi.42.1.228-236.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A. E., Fischinger P. J. Nucleotide sequences in mouse DNA and RNA specific for Moloney sarcoma virus. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3705–3709. doi: 10.1073/pnas.73.10.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A. E., Gilbert J. H., Porzig K. J., Scolnick E. M., Aaronson S. A. Nature and distribution of feline sarcoma virus nucleotide sequences. J Virol. 1979 Jun;30(3):821–827. doi: 10.1128/jvi.30.3.821-827.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B., Rongey R. W., Arnstein P., Estes J. D., Sarma P., Huebner R. J., Rickard C. G. Experimental transmission of feline fibrosarcoma to cats and dogs. Nature. 1970 May 30;226(5248):807–809. doi: 10.1038/226807a0. [DOI] [PubMed] [Google Scholar]
- Ghysdael J., Neil J. C., Vogt P. K. A third class of avian sarcoma viruses, defined by related transformation-specific proteins of Yamaguchi 73 and Esh sarcoma viruses. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2611–2615. doi: 10.1073/pnas.78.4.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. P., Gilboa E., Witte O. N., Baltimore D. Structure of the Abelson murine leukemia virus genome and the homologous cellular gene: studies with cloned viral DNA. Cell. 1980 Dec;22(3):777–785. doi: 10.1016/0092-8674(80)90554-1. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Halpern C. C., Buchhagen D. L., Kawai S. Recovery of avian sarcoma virus from tumors induced by transformation-defective mutants. J Exp Med. 1977 Dec 1;146(6):1735–1747. doi: 10.1084/jem.146.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Wang L. H., Anderson S. M., Karess R. E., Hayward W. S., Hanafusa H. Characterization of the transforming gene of Fujinami sarcoma virus. Proc Natl Acad Sci U S A. 1980 May;77(5):3009–3013. doi: 10.1073/pnas.77.5.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Hayward W. S. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. doi: 10.1128/jvi.24.1.47-63.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itohara S., Hirata K., Inoue M., Hatsuoka M., Sato A. Isolation of a sarcoma virus from a spontaneous chicken tumor. Gan. 1978 Dec;69(6):825–830. [PubMed] [Google Scholar]
- Karess R. E., Hanafusa H. Viral and cellular src genes contribute to the structure of recovered avian sarcoma virus transforming protein. Cell. 1981 Apr;24(1):155–164. doi: 10.1016/0092-8674(81)90511-0. [DOI] [PubMed] [Google Scholar]
- Karess R. E., Hayward W. S., Hanafusa H. Cellular information in the genome of recovered avian sarcoma virus directs the synthesis of transforming protein. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3154–3158. doi: 10.1073/pnas.76.7.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Yoshida M., Segawa K., Sugiyama H., Ishizaki R., Toyoshima K. Characterization of Y73, an avian sarcoma virus: a unique transforming gene and its product, a phosphopolyprotein with protein kinase activity. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6199–6203. doi: 10.1073/pnas.77.10.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. H., Bister K., Pawson A., Robins T., Moscovici C., Duesberg P. H. Fujinami sarcoma virus: an avian RNA tumor virus with a unique transforming gene. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2018–2022. doi: 10.1073/pnas.77.4.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman S. P., Palladino M. A., Thorbecke G. J. Chemical carcinogen-induced transplantable fibrosarcomas in histocompatible chickens. I. Incidence of tumor induction in normal and bursectomized chickens. J Natl Cancer Inst. 1976 Aug;57(2):295–301. doi: 10.1093/jnci/57.2.295. [DOI] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- McDonough S. K., Larsen S., Brodey R. S., Stock N. D., Hardy W. D., Jr A transmissible feline fibrosarcoma of viral origin. Cancer Res. 1971 Jul;31(7):953–956. [PubMed] [Google Scholar]
- Moscovici C., Moscovici M. G., Jimenez H., Lai M. M., Hayman M. J., Vogt P. K. Continuous tissue culture cell lines derived from chemically induced tumors of Japanese quail. Cell. 1977 May;11(1):95–103. doi: 10.1016/0092-8674(77)90320-8. [DOI] [PubMed] [Google Scholar]
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- Neil J. C., Breitman M. L., Vogt P. K. Characterization of a 105,000 molecular weight gag-related phosphoprotein from cells transformed by the defective avian sarcoma virus PRCII. Virology. 1981 Jan 15;108(1):98–110. doi: 10.1016/0042-6822(81)90530-4. [DOI] [PubMed] [Google Scholar]
- Neil J. C., Ghysdael J., Vogt P. K. Tyrosine-specific protein kinase activity associated with p105 of avian sarcoma virus PRCII. Virology. 1981 Feb;109(1):223–228. doi: 10.1016/0042-6822(81)90493-1. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Levinson A. D., Varmus H. E., Levintow L., Bishop J. M. Uninfected vertebrate cells contain a protein that is closely related to the product of the avian sarcoma virus transforming gene (src). Proc Natl Acad Sci U S A. 1979 Apr;76(4):1804–1808. doi: 10.1073/pnas.76.4.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskarsson M., McClements W. L., Blair D. G., Maizel J. V., Vande Woude G. F. Properties of a normal mouse cell DNA sequence (sarc) homologous to the src sequence of Moloney sarcoma virus. Science. 1980 Mar 14;207(4436):1222–1224. doi: 10.1126/science.6243788. [DOI] [PubMed] [Google Scholar]
- Pawson T., Guyden J., Kung T. H., Radke K., Gilmore T., Martin G. S. A strain of Fujinami sarcoma virus which is temperature-sensitive in protein phosphorylation and cellular transformation. Cell. 1980 Dec;22(3):767–775. doi: 10.1016/0092-8674(80)90553-x. [DOI] [PubMed] [Google Scholar]
- Roussel M., Saule S., Lagrou C., Rommens C., Beug H., Graf T., Stehelin D. Three new types of viral oncogene of cellular origin specific for haematopoietic cell transformation. Nature. 1979 Oct 11;281(5731):452–455. doi: 10.1038/281452a0. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Hughes S. H., Kung H. J., Majors J. E., Quintrell N., Guntaka R. V., Bishop J. M., Varmus H. E. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell. 1978 Dec;15(4):1383–1395. doi: 10.1016/0092-8674(78)90063-6. [DOI] [PubMed] [Google Scholar]
- Shibuya M., Hanafusa T., Hanafusa H., Stephenson J. R. Homology exists among the transforming sequences of avian and feline sarcoma viruses. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6536–6540. doi: 10.1073/pnas.77.11.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. P., Theilen G. H. Transmissible feline fibrosarcoma. Nature. 1969 Mar 15;221(5185):1074–1075. doi: 10.1038/2211074a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
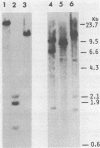
- Spector D. H., Smith K., Padgett T., McCombe P., Roulland-Dussoix D., Moscovici C., Varmus H. E., Bishop J. M. Uninfected avian cells contain RNA related to the transforming gene of avian sarcoma viruses. Cell. 1978 Feb;13(2):371–379. doi: 10.1016/0092-8674(78)90205-2. [DOI] [PubMed] [Google Scholar]
- Spector D. H., Varmus H. E., Bishop J. M. Nucleotide sequences related to the transforming gene of avian sarcoma virus are present in DNA of uninfected vertebrates. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4102–4106. doi: 10.1073/pnas.75.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehelin D., Guntaka R. V., Varmus H. E., Bishop J. M. Purification of DNA complementary to nucleotide sequences required for neoplastic transformation of fibroblasts by avian sarcoma viruses. J Mol Biol. 1976 Mar 5;101(3):349–365. doi: 10.1016/0022-2836(76)90152-2. [DOI] [PubMed] [Google Scholar]
- Stehelin D., Varmus H. E., Bishop J. M., Vogt P. K. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976 Mar 11;260(5547):170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- Van de Ven W. J., Reynolds F. H., Jr, Stephenson J. R. The nonstructural components of polyproteins encoded by replication-defective mammalian transforming retroviruses are phosphorylated and have associated protein kinase activity. Virology. 1980 Feb;101(1):185–197. doi: 10.1016/0042-6822(80)90495-x. [DOI] [PubMed] [Google Scholar]
- Wallbank A. M., Sperling F. G., Hubben K., Stubbs E. L. Isolation of a tumour virus from a chicken submitted to a poultry diagnostic laboratory--Esh sarcoma virus. Nature. 1966 Mar 19;209(5029):1265–1265. doi: 10.1038/2091265a0. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Feldman R., Shibuya M., Hanafusa H., Notter M. F., Balduzzi P. C. Genetic structure, transforming sequence, and gene product of avian sarcoma virus UR1. J Virol. 1981 Oct;40(1):258–267. doi: 10.1128/jvi.40.1.258-267.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Hanafusa H., Notter M. F., Balduzzi P. C. Genetic structure and transforming sequence of avian sarcoma virus UR2. J Virol. 1982 Mar;41(3):833–841. doi: 10.1128/jvi.41.3.833-841.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Y., Hayward W. S., Hanafusa H. Genetic variation in the RNA transcripts of endogenous virus genes in uninfected chicken cells. J Virol. 1977 Oct;24(1):64–73. doi: 10.1128/jvi.24.1.64-73.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Dasgupta A., Baltimore D. Abelson murine leukaemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature. 1980 Feb 28;283(5750):826–831. doi: 10.1038/283826a0. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N. E., Baltimore D. A normal cell protein cross-reactive to the major Abelson murine leukaemia virus gene product. Nature. 1979 Oct 4;281(5730):396–398. doi: 10.1038/281396a0. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Kawai S., Toyoshima K. Genome structure of avian sarcoma virus Y73 and unique sequence coding for polyprotein p90. J Virol. 1981 May;38(2):430–437. doi: 10.1128/jvi.38.2.430-437.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Kawai S., Toyoshima K. Unifected avian cells contain structurally unrelated progenitors of viral sarcoma genes. Nature. 1980 Oct 16;287(5783):653–654. doi: 10.1038/287653a0. [DOI] [PubMed] [Google Scholar]