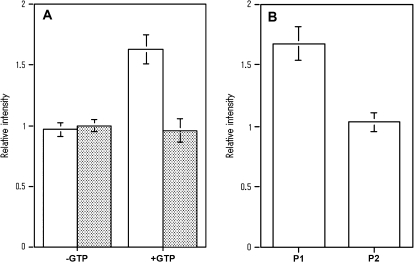
Fig. 4.
Quantitative analysis of His6-AtRab proteins bound to MYA2 tail 2 fused with GST in in vitro binding assays. (A) Binding of His6-AtRab proteins to MYA2 tail 2 coated beads in the absence (–GTP) or presence of GTP (+GTP). White and shaded bars showed the amount of His-AtRabD1 and His-AtRabC2a, respectively, in the pellet with MYA2 tail 2 coated beads. Each His-AtRab protein was mixed with beads in the absence or presence of GTP, or with control beads in the presence of GTP. The amounts of each AtRab protein recovered with beads were determined and are shown as described in the legend to Fig. 3A. Averages ±SD from four separate experiments are plotted. (B) Dissociation of His6-AtRabD1 from MYA2 tail 2 by washing with a buffer containing GTP. His6-AtRabD1 was mixed with MYA2 tail 2 coated beads in the presence or absence of GTP. Washing was performed by the same method as described in the legend to Fig 3B. P1 and P2 show the amounts of His-AtRabD1 recovered in first and second pellets, respectively. Averages ±SD from four separate experiments are plotted.