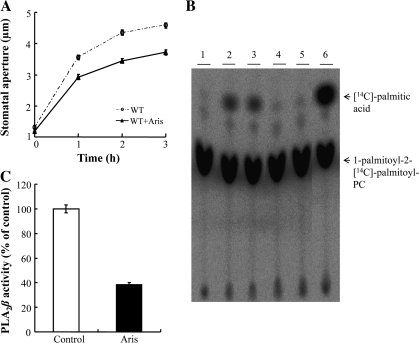
Fig. 4.
The effect of aristolochic acid (Aris), a low molecular weight PLA2 inhibitor, on the activity of purified PLA2β. (A) Light-induced stomatal opening of wild-type Arabidopsis in the presence or absence of 20 μM Aris. (B) Thin layer chromatography (TLC) analysis of the hydrolytic activity of recombinant PLA2β. Purified PLA2β was incubated in 100 mM TRIS–HCl (pH 8.0) at 40 °C in the presence or absence of 20 μM Aris for 40 min, and then PLA2β hydrolytic activity was assayed in the presence of 1-palmitoyl-2-[14C]palmitoyl-PC. The acyl-hydrolysis activity of the recombinant protein was greatly inhibited by Aris, compared to the solvent controls. Lane 1, substrate only; lanes 2–3, solvent controls (40 μM NaOH); lanes 4–5, 20 μM Aris dissolved in NaOH; lane 6, bee venom low molecular weight secretory PLA2 was used instead of purified PLA2β to identify the position of 14C-palmitic acid in the TLC plate. (C) The results from TLC were quantified using a phosphoimager (n=4).