Figure 2.
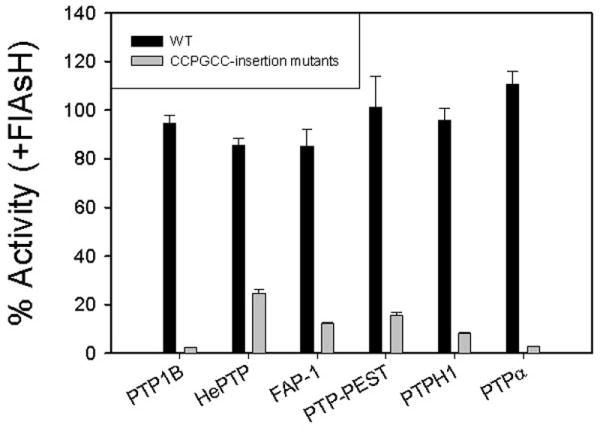
FlAsH-dependent inhibition of PTP-insertion mutants. The indicated PTP enzymes (2.5 μM) were incubated in the absence or presence of FlAsH (10 μM), diluted and assayed for activity with the artificial PTP substrate para-nitrophenyl phosphate (pNPP) at pH 7.0. “% Activity” represents the PTP catalytic efficiency (kcat/KM) in the presence of FlAsH divided by the control (vehicle only) catalytic efficiency of the same enzyme.
