Figure 1.
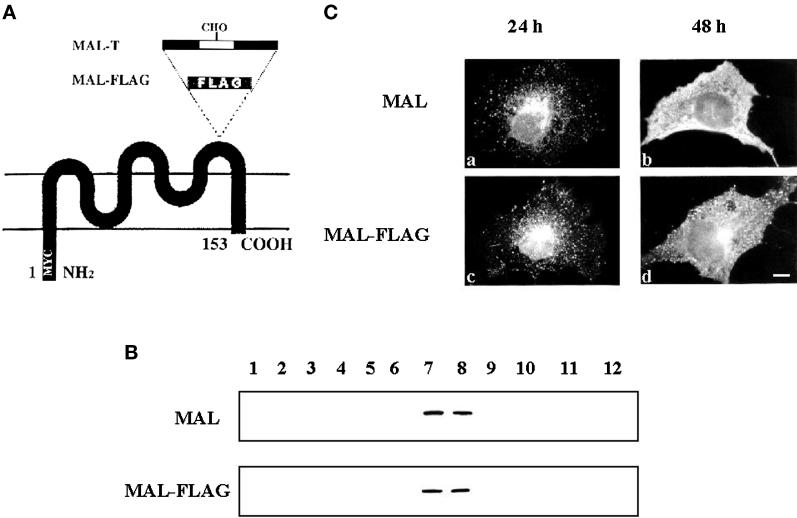
Comparative analysis of MAL and MAL-FLAG expression in COS-7 cells. (A) Schematic diagram of the engineered MAL proteins used in this study. MAL-FLAG was constructed by adding sequences containing the FLAG epitope to the last extracellular loop of MAL. MAL-T was derived from the previously reported MAL-N construct (Puertollano and Alonso, 1999), which contains two consensus N-glycosylation sites in an extended loop, by substituting the asparagine residues within those sites with threonine. (B) To compare the incorporation of MAL and MAL-FLAG into COS-7, cells transiently expressing either MAL or MAL-FLAG were extracted with 1% Triton X-100 at 4°C and subjected to centrifugation to equilibrium in sucrose density gradients at 48 h after transfection. Aliquots from each fraction were subjected to immunoblot analysis with mAb 9E10. Fractions 1–4 are the 40% sucrose layer and contain the bulk of cellular membranes and cytosolic proteins, whereas fractions 5–12 are the 5–30% sucrose layer and contain GEMs. In parallel experiments using MDCK cells, the distribution of calnexin, an endoplasmic reticulum protein that is excluded from GEMs, and caveolin, a GEM resident protein, was used as a control of the fractionation procedure (not shown). (C) The intracellular distribution of MAL (a and b) and MAL-FLAG (c and d) was compared by immunofluorescence analysis with the 9E10 antibody in permeabilized COS-7 cells at either 24 h (a and c) or 48 h (b and d) after transfection. Bar, 10 μm.