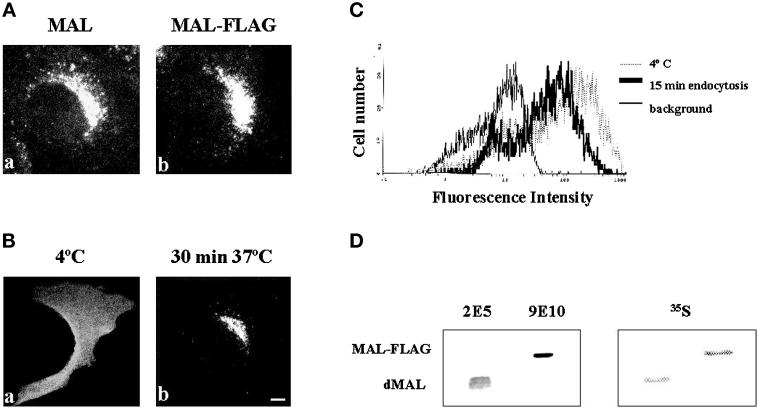
Figure 4.
MAL internalization in stably transfected MDCK cells. (A) MDCK cells stably expressing either MAL (a) or MAL-FLAG (b) were fixed, permeabilized with 0.2% Triton X-100, and subjected to immunofluorescence analysis with mAb 9E10. (B) Intact MDCK cells stably expressing MAL-FLAG were incubated with anti-FLAG antibody for 60 min at 4°C to label surface MAL, washed, and incubated for 30 min at either 4°C, to prevent endocytosis (a), or 37°C, to allow MAL internalization (b). After fixing, cells were permeabilized with 0.2% Triton X-100 and bound antibodies were visualized with fluorescent anti-mouse IgG antibodies. Bar, 10 μm. (C) The experiment in B was repeated omitting the fixation and permeabilization steps. The anti-FLAG antibodies bound to MAL-FLAG remaining at the cell surface after incubation at 37°C for 15 min were analyzed by flow cytometry using a fluorescent secondary antibody. Irrelevant control antibody at 4°C for 75 min (thick line) and anti-FLAG antibodies at 4°C for 60 min followed by 15 min of incubation at either 4°C (thin line) or 37°C (dotted line) are shown. (D) Comparative analysis of the levels of expression of MAL-FLAG and endogenous MAL in transfected MDCK cells. MDCK cells expressing MAL-FLAG were labeled with [35S]methionine/cysteine for 16 h and then extracted with 1% Triton X-100 at 4°C. The GEM fraction was collected and solubilized with 60 mM octyl-glucoside to solubilize GEMs. The solubilized material was then extracted with n-butanol, and the organic phase containing proteolipids was dried and analyzed by SDS-PAGE. Two major proteolipid bands were observed by autoradiography with short exposures. Additional minor bands were detected with longer exposures (not shown). The major bands were identified as endogenous MAL (dMAL) and exogenous MAL-FLAG by immunoblot analysis with antibodies 2E5 and 9E10, respectively. Quantification of the radiolabeled bands indicated that MAL-FLAG expression was approximately twofold higher than that of endogenous MAL.