Figure 2.
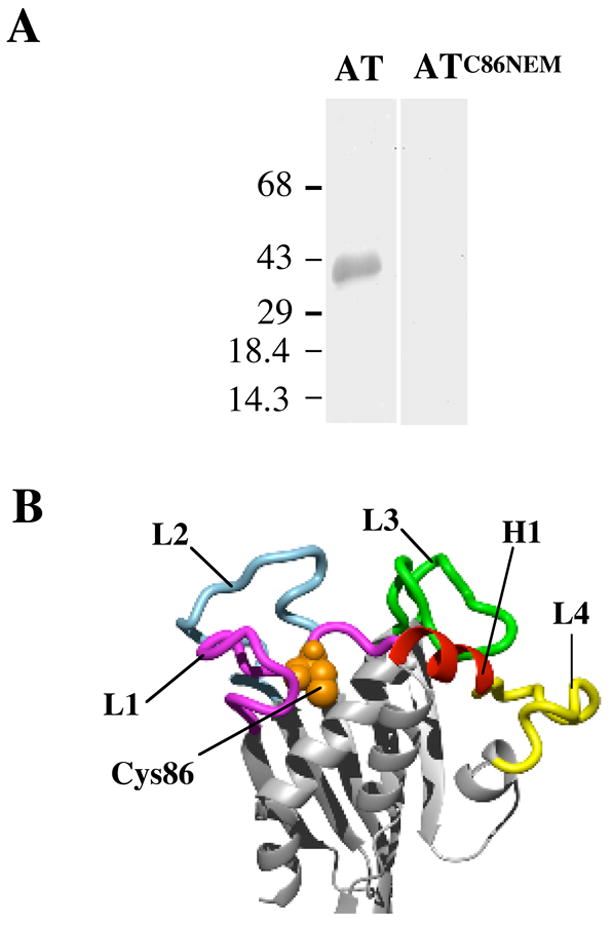
The involvement of D1 in receptor binding. (A) Modification of Cys86 with NEM abolishes binding to hFR. Purified hFR was separated on a 10% SDS-PAGE gel and blotted onto nitrocellulose. Following blocking, the blots were incubated with a solution of 20 nM wild type AT or AT that had been labeled with NEM (ATC86NEM). The blot was then incubated successively with anti-AT antibody and secondary antibody conjugated to alkaline phosphatase. The bands were visualized by the addition of a colorimetric substrate. The molecular mass of the protein markers is in kDa. (B) Molecular model of AT highlighting regions in D1 subjected to alanine scanning. The regions of D1 mutated are color-coded. L1-L4, loops 1–4. H1, helix 1. The position of Cys86 is shown in spacefill.
