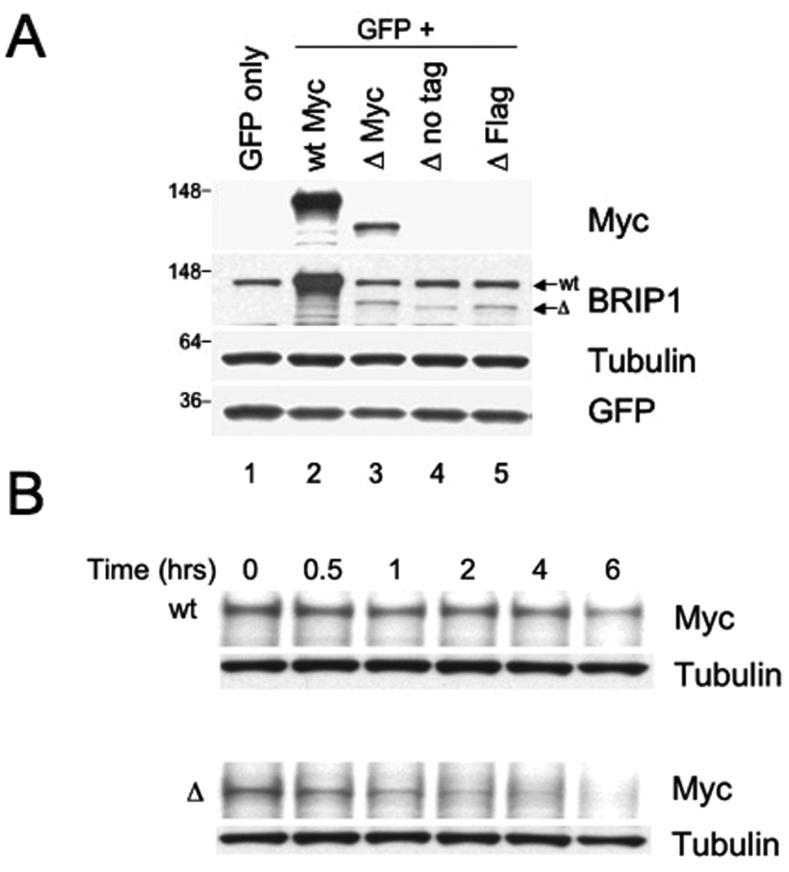
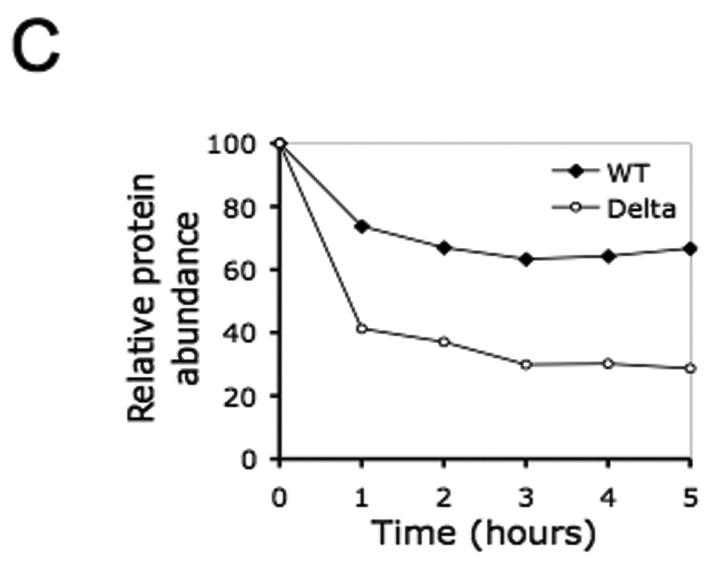
Figure 4. The recombinant ΔBRIP1 is unstable when expressed in 293T cells.
(A) Lysates from 293T cells transfected with a GFP-encoding plasmid alone (lane 1) or in combination with either Myc-wtBRIP1- (lane 2) or Myc-ΔBRIP1-encoding constructs (lanes 3-5) were resolved by SDS-PAGE and probed with the indicated Abs. A significantly reduced abundance of ΔBRIP1 compared to wtBRIP1 was evidenced with both anti-Myc (lane 3 vs lane 2) and anti-BRIP1 (lanes 3-5 vs lane 2) Abs. (B) Cycloheximide-chase analysis of 293T cells expressing Myc-tagged wt or ΔBRIP1 proteins. Equal amounts of lysates, prepared at the indicated time points, were analyzed by western blot with anti-Myc and anti-tubulin Abs. A significant decrease of the ΔBRIP1 signal intensity was detectable already after 1 hour, as opposed to the steady level of wtBRIP1 signal. The experiment was repeated three times with consistent results. (C) Densitometric analysis of a representative cycloheximide chase experiment. Films were scanned and analyzed using the Image J software. The intensity of each band, after normalization to tubulin, which served as loading control, was plotted to determine the half-lives of the wt and mutant BRIP1 proteins.