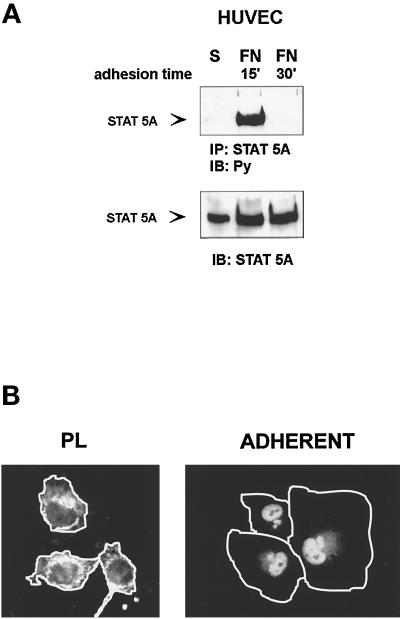
Figure 2.
Time course of adhesion-dependent tyrosine phosphorylation and nuclear translocation of STAT5A. (A) STAT5A activation. Serum-deprived HUVECs were detached with 10 mM EDTA and plated on FN-coated dishes for the indicated times or kept in suspension (S). Cell extracts were immunoprecipitated (IP) with antibodies to STAT5A protein and subjected to 8% SDS-PAGE. Proteins were electrophoretically transferred to nitrocellulose filter and immunoblotted (IB) with an anti-phosphotyrosine antibody (Py) (upper panel). The filter was stripped and reprobed with the anti-STAT5A antiserum (lower panel). Migration of the tyrosine-phosphorylated STAT5A is indicated by the arrow. (B) STAT5A nuclear translocation. Serum-deprived HUVECs were detached and plated on FN-coated glass coverslips for 60 min. (ADHERENT) and then fixed in 3% paraformaldehyde in PBS for 10 min. As control, cells were plated for the same time on PL. After permeabilization cells were stained for 1 h at room temperature with STAT5A antibodies, washed, and exposed to a secondary FITC-labeled antibody for 1 h. Coverslips were then mounted in PBS:glycerol, 1:1, viewed on Olympus (Tokyo, Japan) BH2-RFCA fluorescence microscope, and photographed. Photographs were then processed by Adobe (Mountain View, CA) Photo De Luxe 2.0 to magnify cell shape. White lines define the boundaries of the cells.