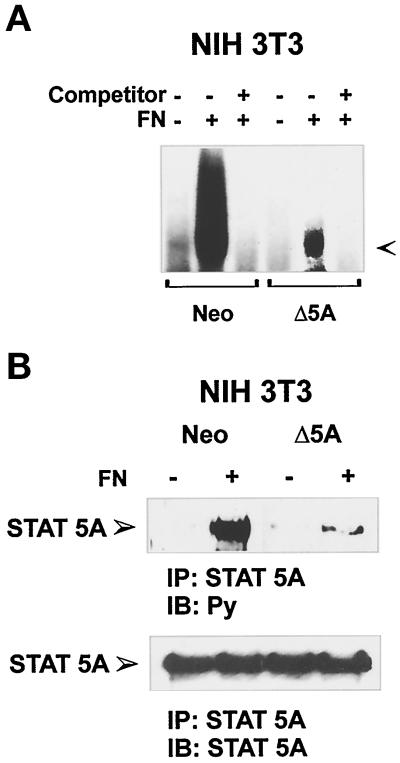
Figure 5.
FN-mediated STAT5A activation in NIH3T3 cells ectopically transfected with Neo vector or a dominant negative STAT5A construct. (A) PIE-binding complex. NIH3T3 cells ectopically transfected with a Neo selection marker (Neo) or dominant negative STAT5A cDNA (Δ5A) were plated for 40 min on FN (+) or kept in suspension (−), and nuclear extracts were prepared. Thirty minutes before the addition of radiolabeled PIE oligonucleotides, the indicated extracts were treated with 50-fold excess of unlabeled oligonucleotide (Competitor). The DNA–protein complexes were then resolved by nondenaturing PAGE. The PIE complex is indicated by the arrow. (B) Effect of the dominant negative STAT5A expression on FN-mediated activation of the endogenous STAT5A. Cell extracts from NIH3T3 ectopically transfected with a Neo selection marker (Neo) or dominant negative STAT5A cDNA (Δ5A) plated on FN-coated dishes for 15 min or kept in suspension (S) were prepared and immunoprecipitated (IP) with antibodies to STAT5A protein. Proteins were subjected to 8% SDS-PAGE, electrophoretically transferred to a nitrocellulose filter, and immunoblotted (IB) with an anti-phosphotyrosine antibody (Py) (upper panel). The filter was stripped and reprobed with the anti-STAT5A antiserum (lower panel). Migration of the tyrosine-phosphorylated STAT5A is indicated by the arrow.