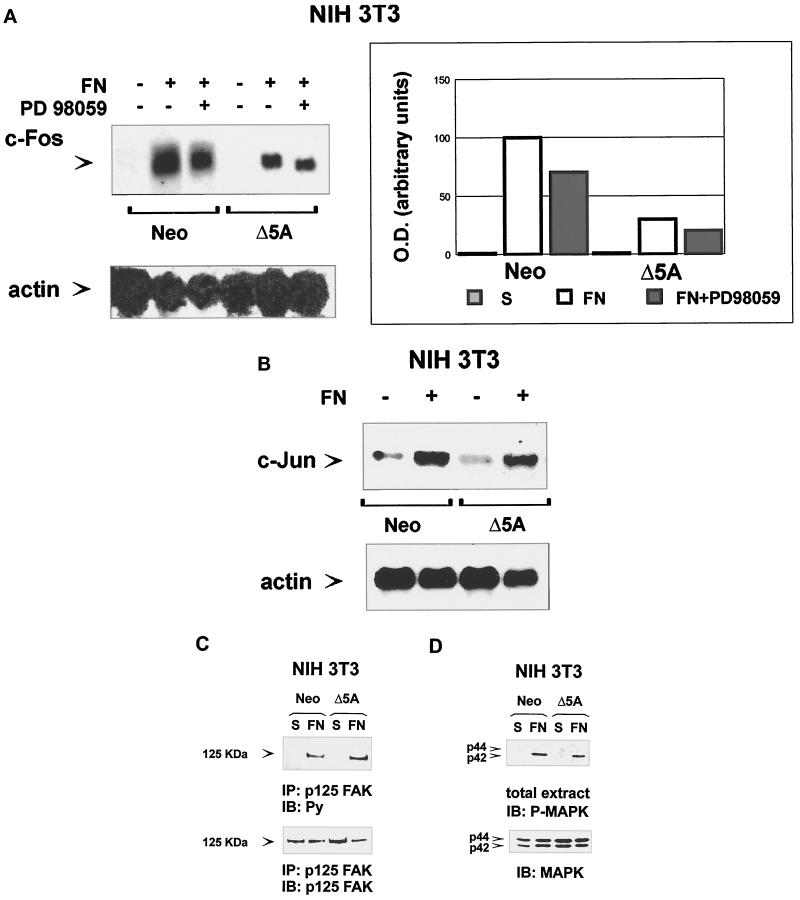
Figure 6.
Expression of dominant-negative STAT5A reduces integrin-mediated c-fos gene expression without interfering with integrin-induced p125FAK and Erk1/Erk2 MAP kinase phosphorylation. (A and B) Integrin-mediated c-fos and c-jun gene expression. Serum-deprived NIH3T3 fibroblasts ectopically transfected with Neo vector (Neo) or with a dominant negative STAT5A cDNA (Δ5A) were kept in suspension (−) or plated on FN-coated dishes (+) for 1 h in the presence or in the absence of the MAP–extracellular signal-regulated kinase kinase inhibitor PD98059 (+) as indicated. Total cytoplasmic RNA was isolated by acid phenol-chloroform extraction. Northern blot analysis was performed according to standard methods, and filters were hybridized with a specific mouse c-fos (A, upper panel, left), c-jun (B, upper panel), or β actin (lower panels) cDNA probes. Mouse c-fos hybridization was also quantified by densitometric analysis (A, right) using a Bio-Rad GS 250 molecular imager. (C and D) Integrin-dependent p125FAK and Erk1/Erk2 MAP kinase phosphorylation. Serum-deprived NIH3T3 cells ectopically transfected with a Neo selection marker (Neo) or dominant negative STAT5A cDNA (Δ5A) were processed as above and plated on FN-coated dishes for 15 min or kept in suspension (S). Cells were lysed and either immunoprecipitated (IP) with an anti-p125FAK antiserum or run on 8% SDS-PAGE. Proteins were electrophoretically transferred to nitrocellulose filters that were immunoblotted (IB) with an anti-phosphotyrosine antibody (C) or with an anti-phospho-Erk1/Erk2 MAP kinase antibody (D, upper panels) and reprobed with the anti-p125FAK or with an anti-Erk1 MAP kinase antibody (lower panels).