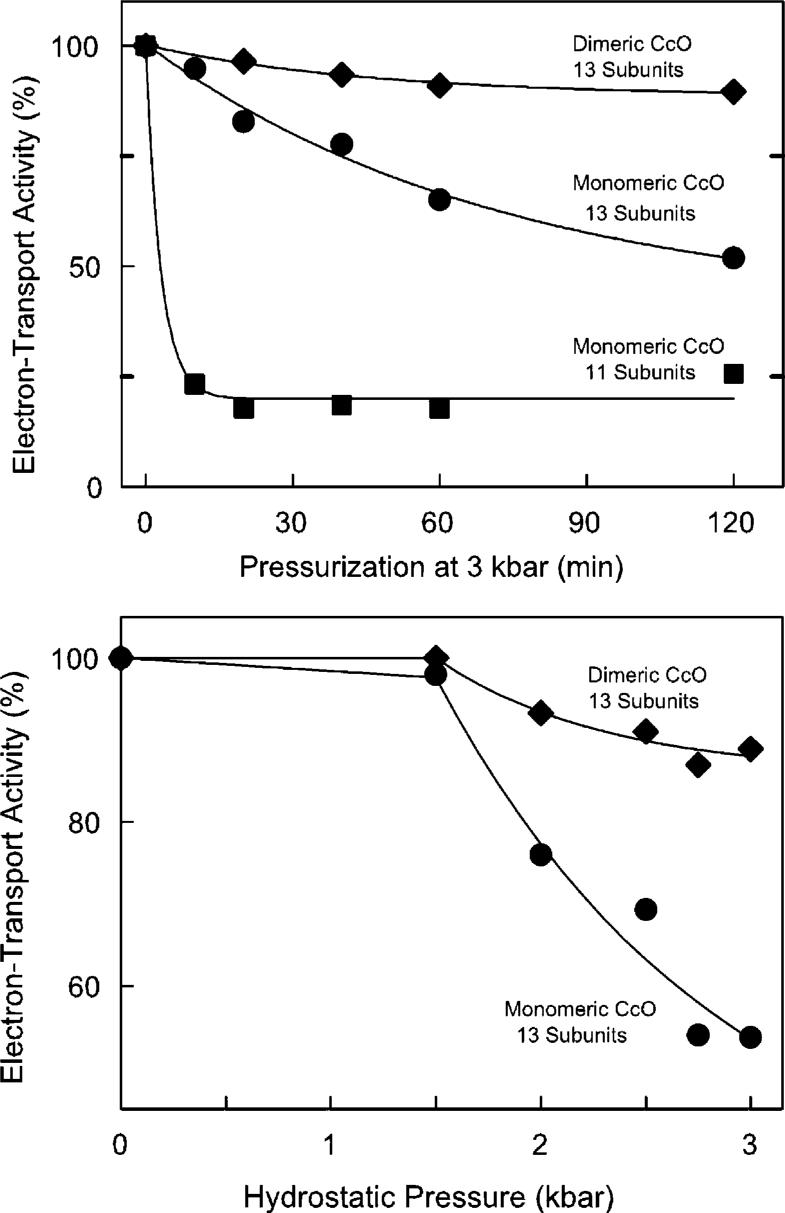
Figure 1.
Hydrostatic pressure-induced inactivation of cytochrome c oxidase. The electron transfer activity of dimeric (◆), 13-subunit monomeric (◆), and 11-subunit monomeric CcO (■) was measured as a function of exposure time to 3 kbar of hydrostatic pressure (top) and as a function of hydrostatic pressure treatment for 2 h (bottom). The three data sets were fitted with single-term exponentials. The fitting parameters at infinite time were 88, 40, and 20% for dimeric, monomeric, and 11-subunit CcO, respectively. In each case, 8 μM aa3 was exposed to hydrostatic pressure at 25 °C in 20 mM Tris-SO4 buffer (pH 7.4) containing the following amount of detergent: 2 mM DM and 2 mM sodium cholate for dimeric CcO; 10 mM DM for 13-subunit, monomeric CcO; and 2 mM DM for 11-subunit CcO. Electron transfer activity of each sample was measured after decompression and dilution into dodecyl maltoside-containing assay buffer. Data are reproducible to ±5% based upon more than a dozen repetitions of each pressure-induced inactivation curve using four different preparations of CcO.