Fig. 4. Generation of full-length peptide lacking a glycine hinge.
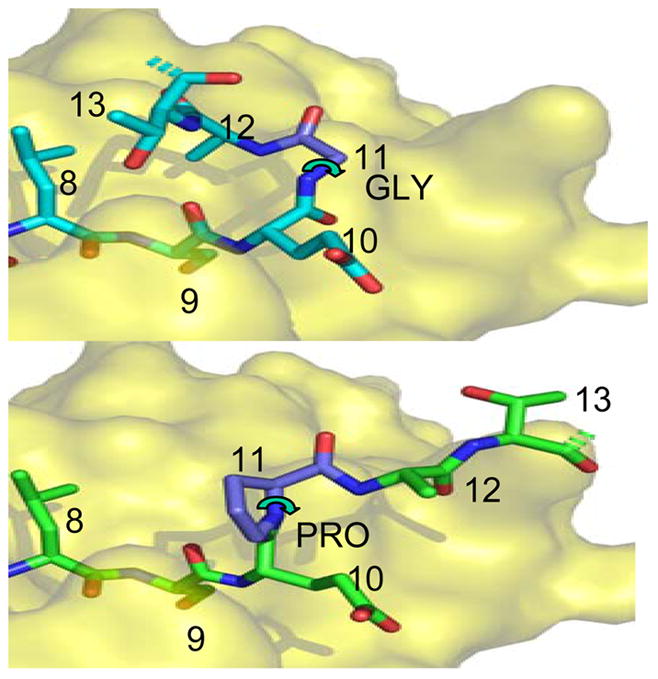
Top: Structure of native gag PP16 peptide bound to HLA-DR1, from the crystal structure of the complex (Zavala-Ruiz et al., 2004). The view shows the C-terminal region of the peptide binding site, viewed from above. The dashed bonds indicate the continuation of the peptide backbone to the terminal proline residue; this was not visualized in the crystal structure. The P9 residue serine is buried in the site, and the other residues are partially or completely exposed. The glycine at P11 allows the peptide backbone to bend sharply, forming a hairpin that orients the P13 residue threonine for interaction with TCR.
Bottom: Hypothetical model of the Pro11 peptide bound into the same site, with the proline at P11 indicated in a common conformation. Note that this peptide cannot assume the hairpin conformation shown above because the proline 5-membered ring severely constrains the phi angle (curved arrow) to ~60 degrees. This angle is free to rotate for glycine.
