Abstract
Informative and tractable animal models that are colonized by well-defined microbial pathogens represent ideal systems for the study of complex human diseases. Helicobacter pylori colonization of the stomach is a strong risk factor for peptic ulceration and distal gastric cancer. However, gastritis has no adverse consequences for most hosts and emerging evidence suggests that H. pylori prevalence is inversely related to gastroesophageal reflux disease and allergic disorders. These observations indicate that eradication may not be appropriate for certain populations due to the potentially beneficial effects conferred by persistent gastric inflammation. Animal models have provided an invaluable resource with which to study H. pylori pathogenesis and carcinogenesis, and have permitted the development of a focused approach to selectively target human populations at high-risk of disease.
Helicobacter pylori is a bacterium that colonizes gastric epithelium and represents the most common bacterial infection worldwide (Peek and Blaser, 2002). H. pylori has colonized human stomachs for over 58,000 years (Linz et al., 2007), and virtually all persons infected by this organism develop co-existing gastritis, a signature feature of which is the capacity to persist for decades. Owing to its co-evolution with humans, H. pylori can send and receive signals from gastric epithelium, allowing host and bacteria to participate in a dynamic equilibrium. However, there are biological costs to these long-term relationships.
Epidemiological studies in humans and experimental infections using a variety of animal models have clearly demonstrated that sustained interactions between H. pylori and its host significantly increase the risk for peptic ulcer disease, distal gastric adenocarcinoma, and non-Hodgkin’s lymphoma of the stomach (Peek and Blaser, 2002). Eradication of H. pylori significantly decreases the risk of developing peptic ulceration or gastric adenocarcinoma in infected individuals without pre-malignant lesions (Wong et al., 2004), providing evidence that this organism influences early stages in gastric carcinogenesis. However, only a fraction of colonized persons ever develop ulcers or neoplasia, and disease risk involves well-choreographed interactions between pathogen and host, which, in turn, are dependent upon strain-specific bacterial factors and/or host characteristics.
Microbial mediators of disease
H. pylori strains isolated from different individuals are extremely diverse and we have previously demonstrated that genetically unique derivatives of a single strain are present simultaneously within an individual human host, and that the genetic composition of isolates can change over time (Israel et al., 2001a). The ability of H. pylori to readily take up and integrate exogenous DNA into its chromosome (competence) contributes to the generation of such diversity, a property that has been co-opted by investigators to examine effects of bacterial gene products on pathogenic host responses.
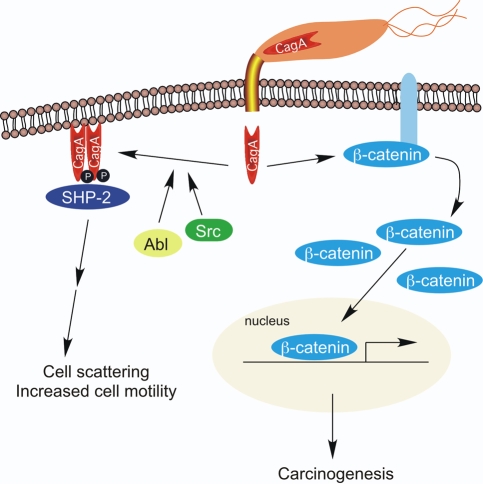
One microbial determinant that augments disease risk is the cag pathogenicity island, which is present in approximately 60% of US strains. H. pylori cag+ strains significantly increase the risk of peptic ulceration and distal gastric cancer compared with strains that lack the cag island (Peek and Blaser, 2002). Several cag genes encode products that form a type IV bacterial secretion system, which translocates the product of the terminal gene in the island (CagA) into host epithelial cells after bacterial attachment (Fig. 1). Intracellular CagA undergoes tyrosine phosphorylation by Src and Abl kinases and activates a eukaryotic phosphatase (SHP-2) as well as ERK, a member of the mitogen-activated protein kinase (MAPK) family, leading to morphological changes that are reminiscent of unrestrained stimulation by growth factors (Fig. 1). Non-phosphorylated CagA also exerts effects within the cell, such as aberrant activation of β-catenin and disruption of apical-junctional complexes: alterations that play a role in carcinogenesis (Amieva et al., 2003; Franco et al., 2005). Recent studies have now firmly implicated this effector as a bacterial oncoprotein by demonstrating that CagA can attenuate apoptosis and that transgenic expression of CagA in mice leads to the development of gastric carcinoma (Mimuro et al., 2007; Ohnishi et al., 2008).
Fig. 1. Molecular signaling alterations induced by intracellular delivery of CagA.
After bacterial attachment, the cag secretion system translocates CagA, the product of the terminal gene in the cag island, into host epithelial cells. Intracellular CagA undergoes tyrosine phosphorylation by Src and Abl kinases, and activates a eukaryotic phosphatase (SHP-2) leading to morphological changes that are reminiscent of unrestrained stimulation by growth factors. Non-phosphorylated CagA also exerts effects within the cell, such as aberrant activation of β-catenin, leading to targeted, transcriptional upregulation of genes implicated in carcinogenesis.
vacA, which encodes a secreted bacterial toxin (VacA), is another H. pylori locus linked with disease and strains vary in cytotoxin activity as a result of variations in vacA gene structure. The regions of greatest diversity are localized near the 5′ end of vacA (allele types s1a, s1b, s1c, or s2), the mid-region of vacA (allele types m1 or m2), or the intermediate region (allele types i1 or i2) (Rhead et al., 2007). H. pylori strains that possess s1/m1/i1 vacA alleles are associated with an increased risk of gastric cancer compared with vacA s2/m2/i2 strains (Rhead et al., 2007). When added to polarized epithelial cell monolayers, VacA induces apoptosis and increases paracellular permeability to organic molecules, iron and nickel (Cover et al., 2003). VacA has also been shown to actively suppress T-cell proliferation and activation in vitro (Gebert et al., 2003), which may contribute to the longevity of H. pylori colonization.
Several different host receptors for H. pylori have been identified, including decay accelerating factor (DAF), which regulates the intensity of gastritis in response to this pathogen (O’Brien et al., 2006). Sequence analysis of the genomes from the completely sequenced H. pylori strains 26695, J99 and HPAG1 has revealed that a high proportion of identified open reading frames are predicted to encode outer membrane proteins (OMPs). OMPs can function as adhesins and permit H. pylori to engage in a range of interactions with host cells, some of which play a role in pathogenesis. The H. pylori adhesin SabA binds sialyl-Lewisx, an established host tumor antigen and marker of gastric dysplasia (Mahdavi et al., 2002). BabA, encoded by babA2, binds the Lewisb (Leb) antigen on gastric epithelial cells (Ilver et al., 1998) and carriage of H. pylori strains that possess babA2 increase the risk of gastric cancer. The presence of babA2 is associated with cagA and vacA s1 alleles, and strains that possess all three of these genes incur the highest risk of gastric cancer (Gerhard et al., 1999).
Host constituents that mediate H. pylori-induced injury
In addition to H. pylori components, polymorphisms within the human IL-1βgene promoter that permit increased expression of IL-1β (a pro-inflammatory cytokine with potent acid-suppressive properties), heighten the risk of gastric adenocarcinoma (El-Omar et al., 2000). These relationships are present only among H. pylori-colonized persons, emphasizing the importance of host-environment interactions in the progression to gastric cancer. High-expression TNF-αpolymorphisms as well as polymorphisms that reduce the production of anti-inflammatory cytokines, such as IL-10, also increase the risk of gastric cancer (El-Omar et al., 2003). The combinatorial effect of these polymorphisms on cancer risk is synergistic, such that three polymorphisms increase the risk of cancer 27-fold over baseline (El-Omar et al., 2003). Polymorphisms in pattern recognition receptors such as Toll-like receptor 4 (TLR-4) have also been linked with an enhanced susceptibility to H. pylori-induced gastric cancer (Hold et al., 2007). Among persons with high-risk IL-1βpolymorphisms who are also colonized by H. pylori cag+ or toxigenic strains, the relative risks of gastric cancer are further augmented to 25- and 87-fold over baseline, respectively (Figueiredo et al., 2002). This indicates that interactions between specific host and microbial determinants are biologically significant for the development of gastric cancer.
Animal models to investigate the role of H. pylori virulence constituents
Determination of the full contribution of the host microenvironment to H. pylori-induced gastric cancer necessitates the use of animal models, and such systems have provided valuable insights into the host, bacterial and environmental factors involved in gastric carcinogenesis. Rodents and primates are the primary models that have been used and although each model has its own distinct advantages and disadvantages, they should be viewed as complementary systems. Mice are inbred, permitting host variables to be carefully controlled. Most mouse strains do not develop cancer but only mild inflammation following H. pylori infection. Alternatively, Mongolian gerbils are outbred and are not as useful as mice for the study of host factors, but gerbils can develop cancer when colonized with certain strains of H. pylori. Primates are the most closely related of these models to the human host, but experimental manipulations in monkeys cannot be conducted on the same scale as rodents and, therefore, large studies are impractical because of the high costs.
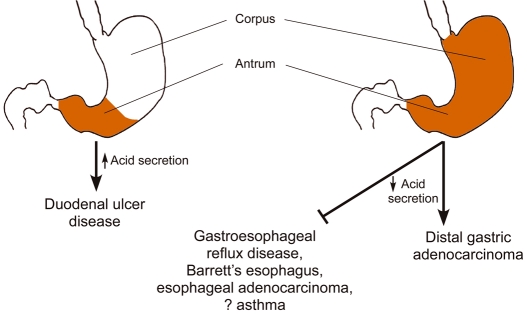
Components of the H. pylori cag island induce epithelial responses in vitro that are linked with carcinogenesis. However, in vivo studies using mice have not readily recapitulated these observations and infection of wild-type mice with cag+ strains frequently leads to deletions within the cag island (Sozzi et al., 2001; Philpott et al., 2002). By contrast, H. pylori reproducibly induces gastric inflammation in gerbils, and various H. pylori mutant strains colonize this model well (Peek et al., 2000; Israel et al., 2001b), which allows an examination of the role of virulence determinants on parameters of gastric injury. Compared with gerbils infected with wild-type H. pylori, gerbils colonized with cag island mutant strains develop significantly less-severe gastritis (Ogura et al., 2000; Israel et al., 2001b). Rieder et al. investigated alterations not only in the intensity but also the topography of inflammation in gerbils infected with wild-type H. pylori or isogenic cagA or cagY (secretion system deficient) mutant derivatives (Rieder et al., 2005). Loss of cagA or cagY resulted in an inflammatory response that was primarily restricted to the gastric antrum, and which did not significantly involve the acid-secreting corpus. Consistent with these histologic changes, intragastric pH values were increased only in gerbils challenged with the wild-type H. pylori strain (Rieder et al., 2005). These results indicate that a functional cag secretion system is required to induce corpus-predominant gastritis, a precursor lesion in the progression to gastric adenocarcinoma (Fig. 2).
Fig. 2. Relationships between the location of H. pylori-induced gastric inflammation, acid secretion, and disease.
Antral-predominant gastritis with relative sparing of the acid-secreting corpus leads to increased acid secretion and an increased risk for duodenal ulcer disease. Corpus-predominant gastritis, a precursor lesion in the progression to gastric adenocarcinoma, is associated with reduced acid secretion and may represent a factor underpinning the inverse association between H. pylori infection and complications of gastroesophageal reflux disease.
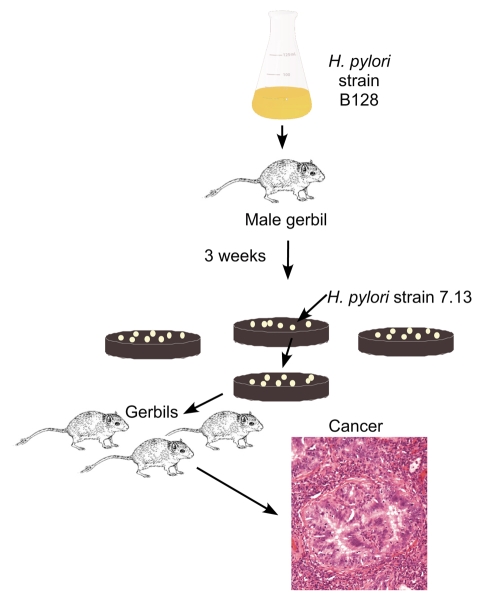
H. pylori not only induces lesions with pre-malignant potential in gerbil gastric mucosa, but long-term infection can also lead to gastric adenocarcinoma, without the co-administration of known carcinogens (Honda et al., 1998; Watanabe et al., 1998; Ogura et al., 2000; Zheng et al., 2004). However, the prolonged time-course required for transformation has precluded large-scale analyses that evaluate effects of both pathogen and host in the carcinogenic cascade. Since serial passage of H. pylori in rodents increases colonization efficiency, our laboratory investigated whether in vivo adaptation of a human H. pylori strain (B128) would enhance its carcinogenic potential. A gerbil infected with H. pylori strain B128 was sacrificed 3 weeks post-challenge, and a single colony output derivative (7.13) was used to infect an independent population of gerbils (Fig. 3) (Franco et al., 2005). The kinetics and intensity of inflammation induced by strain 7.13 were similar to those induced by strain B128; however, gastric dysplasia and adenocarcinoma developed by 8 weeks in approximately 75% and 60% of gerbils infected with strain 7.13, respectively, whereas these lesions were not present in any gerbil infected with the progenitor H. pylori strain B128 (Franco et al., 2005). We recently extended these results by infecting gerbils with wild-type strain 7.13 or a 7.13 cagA– mutant and demonstrated that the development of gastric cancer in this model is dependent upon CagA (Franco et al., 2008).
Fig. 3. In vivo derivation of carcinogenic H. pylori strain 7.13.
A gerbil infected with the human H. pylori clinical isolate B128 was sacrificed 3 weeks post-challenge, and a single colony output derivative (7.13) was used to infect an independent population of gerbils. Gerbils infected with strain 7.13, but not B128, developed gastric carcinoma.
Compared with gerbils, wild-type mice are not as susceptible to H. pylori-induced injury, which has forced modifications to be made from both the microbial and the host side to optimize murine models of inflammation-induced gastric cancer. To circumvent the inability of H. pylori to induce a robust inflammatory response in mouse gastric mucosa, the murine pathogen Helicobacter felis has been used to induce gastric injury, and the degree of gastric damage is usually more severe in mice infected with H. felis compared with H. pylori. Gastric adenocarcinoma can also develop following long-term infection of wild-type C57/Bl6 mice with H. felis (Cai et al., 2005). However, many H. pylori virulence components, such as the cag pathogenicity island and vacA, are not present within the genome of H. felis, which limits the usefulness of this system to study interactions between clinically important H. pylori constituents and the induced host response.
From the host side, transgenic mice have been generated that are more susceptible to gastric cancer than wild-type strains, and these strains have provided insights into host factors that mediate gastric carcinogenesis. Several of these models can develop gastric cancer in the absence of H. pylori infection, including mice that are genetically deficient for trefoil factor 1 (TFF1), Smad4, RUNX3 and gastrin, which leads to chronic atrophic gastritis (Peek and Crabtree, 2006). Mutation of the IL-6 family co-receptor gp130 leads to altered SHP-2 signaling and constitutive activation of STAT3, which also culminates in the development of intestinal-type gastric adenocarcinoma in genetically engineered mice in the absence of infection (Tebbutt et al., 2002; Judd et al., 2004).
One host determinant that may influence the development of gastric cancer is gastrin and, in vitro, gastrin stimulates gastric epithelial cell proliferation (Iwase et al., 1997). Similarly to gastrin-deficient mice, transgenic mice that overexpress gastrin (INS-GAS mice) spontaneously develop gastric cancer, but this requires the virtual lifetime of the animal (Wang et al., 2000). Concomitant infection with the mouse-adapted H. pylori strain SS1 or the gerbil-adapted H. pylori strain 7.13 accelerates this process (Fox et al., 2003a; Fox et al., 2003b), which suggests that persistently elevated gastrin levels synergize with H. pylori to augment the progression to gastric cancer. A study that used the INS-GAS model of gastric cancer demonstrated that inactivation of cagE, which encodes a component of the cag secretion apparatus, temporally delayed, but did not prevent, the development of cancer in H. pylori-infected mice (Fox et al., 2003b).
In addition to mice and gerbils, primates have been used to examine the role of cag genes on induction of disease. One study used H. pylori-infected Rhesus monkeys to examine expression of genes within the cag island by quantitative real-time reverse transcriptase PCR (Boonjakuakul et al., 2005). These data indicated that cagA was expressed at high levels during the entire time course of infection. Interestingly, some cag genes, such as cagY, were more highly expressed at 1 week post-infection compared with later time points, whereas expression of others, such as cagC, increased between 2 and 3 months and then fell by 4–6 months post-challenge (Boonjakuakul et al., 2005). Thus, data obtained from these independent animal model systems indicate an important role for CagA and other products of the cag pathogenicity island in the development of H. pylori-induced disease, particularly gastric cancer.
Similarly to studies focused on the cag island, less is known about the function of VacA in vivo when compared with in vitro observations. Multiple studies have been unable to detect a significant difference in levels of injury induced by wild-type versus vacA mutant H. pylori strains in various animal models (Eaton et al., 1997; Wirth et al., 1998; Guo and Mekalanos, 2002), although one study demonstrated that a vacA mutant was less efficient than wild-type H. pylori in colonization of mice (Salama et al., 2001). Experiments using purified VacA have yielded different results. The mid-region of VacA contains a cell-binding site; m1-type toxins exhibit higher binding affinities to host cells than do m2-type toxins, and VacA has been demonstrated to bind to a unique receptor-type protein tyrosine phosphatase, PTPζ, a member of a family of receptor-like enzymes that regulate cellular proliferation, differentiation and adhesion (Fujikawa et al., 2003). Oral delivery of purified VacA induced gastric inflammation, hemorrhage and ulcers, but only in PTPζ+/+ mice and, in vitro, VacA treatment of PTPζ+/+, but not PTPζ−/−, cells induced cellular detachment, which may contribute to H. pylori-induced ulcerogenesis in humans (Fujikawa et al., 2003).
Adherence plays an important role in the induction of pathologic sequelae and several H. pylori adhesins have been studied in animal models. Studies in which Rhesus macaques were experimentally infected with H. pylori have demonstrated that the gene encoding BabA (babA2) could be replaced with the highly related gene babB via recombination (Solnick et al., 2004). As expected, recovered isolates that did not express BabA were deficient in their ability to bind Leb when tested in vitro, indicating that H. pylori can use antigenic variation to regulate its interaction with host cells.
Animal models were instrumental for identifying the H. pylori adhesin SabA, which binds the sialylated glycan sialyl-LeX (Mahdavi et al., 2002). Using gastric biopsy tissue from a Rhesus monkey, Mahdavi et al. demonstrated that, in situ, an H. pylori BabA mutant was still able to bind gastric epithelium, and the topography of binding reflected the expression of sialyl-LeX. Similarly, when H. pylori were pretreated with sialyl-LeX, the ability of this mutant strain to bind sialyl-LeX-expressing gastric tissue was reduced >90% compared with the wild-type strain. Finally, experimental infection of a monkey with a sialyl-LeX-binding H. pylori strain revealed that H. pylori increases expression of sialyl-LeX in the gastric epithelium (Mahdavi et al., 2002). Collectively, these results indicate that in vivo studies using animal models for the study of H. pylori virulence constituents will continue to be a fertile area of research. Additional need for the development and characterization of appropriate model organisms for H. pylori is becoming apparent as new evidence arises suggesting some beneficial effects associated with infection.
Potential benefits conferred by chronic H. pylori colonization
Although H. pylori represents a significant risk factor for serious diseases of the upper gastrointestinal tract, the epidemiology of this pathogen is changing rapidly, particularly in developed countries. H. pylori is present in approximately 10% of children in the USA under age 10, compared with 50–60% of persons greater than 60-years old (Peek and Blaser, 2002). Since the rate of acquisition of H. pylori among adults in this country is <1% per year, most currently colonized adults likely acquired their infections during childhood. The progressive decline in H. pylori acquisition during the last century in the USA has been mirrored by an expected decrease in the incidence of peptic ulceration and distal gastric cancer, but these changes have been diametrically opposed by a rapidly increasing incidence of gastroesophageal reflux disease (GERD) and its sequelae, which include Barrett’s esophagus and esophageal adenocarcinoma (Peek and Blaser, 2002). Among white males, the incidence of esophageal adenocarcinoma has increased more than 350% since 1975 and its incidence is rising more rapidly than any other malignancy in this country. The relatively short time-frame over which the frequency of this cancer has increased suggests that an environmental factor may be involved. Could this factor be the loss of H. pylori?
Several studies performed in geographically distinct regions of the world have demonstrated that carriage of H. pylori is associated with a significantly reduced risk of developing GERD, Barrett’s esophagus and esophageal adenocarcinoma. This reciprocal effect is almost entirely attributable to the presence of strains harboring the cag pathogenicity island (Peek and Blaser, 2002). Although the precise mechanisms through which cag+ strains decrease the risk for GERD remain speculative, location of inflammation within the gastric niche is probably crucial. Most physiological studies indicate that patients with antral-predominant gastritis have increased levels of gastric acidity, while acid secretion rates are attenuated in H. pylori+ subjects with pan-gastritis (Fig. 2). By inhibiting parietal cell function and/or accelerating the development of atrophic gastritis, enhanced inflammation induced by cag+ strains within the acid-secreting gastric corpus may blunt the levels of acid secretion that are required for the development of GERD and its sequelae. Consistent with this hypothesis, severe corpus gastritis, atrophic gastritis, and decreased acid production induced by chronic H. pylori colonization are associated with a significantly reduced risk of GERD.
In addition to GERD, there has been a recent increase in the prevalence of asthma, allergic rhinitis and atopy in industrialized nations (Chen and Blaser, 2007). The disappearance of H. pylori has preceded the rise in the prevalence of these conditions, prompting investigators to examine the relationship between H. pylori infection and allergic disorders from a clinical perspective. Several cross-sectional or case control studies by multiple investigators have demonstrated significant inverse relationships are present between H. pylori infection and asthma, atopy allergic rhinitis, and/or eczema (reviewed by Blaser et al., 2008). This relationship is similar to the pattern between H. pylori infection and GERD and its sequelae. Further, these reciprocal relationships are most pronounced in young persons infected with cag+ strains (Blaser et al., 2008). Although these studies lack a prospective design, there are potential mechanisms through which H. pylori may reduce the risk of asthma and associated allergic conditions. Hypochlorhydric conditions that mediate a decreased risk for GERD (described above) may also explain the inverse relationship between H. pylori and allergy since a substantial fraction of asthma cases, especially in adults, are due to GERD (Farrokhi and Vaezi, 2007; Blaser et al., 2008). H. pylori manipulates the host immune response, including the activation of T regulatory cells, which may, in turn, dampen immunomodulatory activities against environmental allergens. Although the precise mechanisms remain undefined, current data indicate that the interaction of H. pylori cag+ strains with their hosts confers opposing risks for important diseases, underscoring the importance of identifying colonized individuals who are at enhanced risk for pathologic outcome, for whom diagnosis and therapy is warranted.
The inverse association between infection with H. pylori and host allergic responses is provocative and has increased interest in this field. A subpopulation of investigators and clinicians believe eradication of H. pylori is in the best public health interest due to the positive correlation between infection and peptic ulcers or cancer. It is difficult, however, to place the potential beneficial effects of this infectious agent into the context of common current paradigms. Further, the mechanisms induced by H. pylori that provide protection against other unwanted forms of inflammation are not known and this understanding will be necessary to provide molecular validation of the inverse relationship between GERD, asthma and H. pylori infection. As allergy and asthma become increasingly common in the western world, the importance of understanding how they are influenced by infectious agents, such as H. pylori and possibly others, is of central importance to human health. Thus, the association between H. pylori infection and reduced risks of asthma and allergy provide a rich new area that will benefit from research of host responses in well-characterized model organisms.
Conclusions
Establishment of H. pylori as a risk factor for peptic ulceration and gastric cancer permits an approach to identify persons at increased risk; however, infection with this organism is extremely common and most colonized persons never develop disease. Chronic infection may also be beneficial by limiting the development of other important diseases, which suggests that H. pylori is actually an amphibiont: a microbial species that functions as a pathogen or a symbiont, depending on the specific context (Blaser et al., 2008). Thus, techniques to identify sub-populations at high risk of disease must use other biological markers than simply detection of H. pylori. It is apparent from recent studies that gastric cancer risk is the summation of the polymorphic nature of the bacterial population in the host, the host genotype, and environmental exposures, each affecting the level of long-term interactions between H. pylori and humans. Analytical tools now exist, however, including genome sequences (H. pylori and human), easily transformable H. pylori strains, measurable phenotypes, and practical animal models, to discern the fundamental biological basis of H. pylori-associated neoplasia, which should have direct clinical applications.
In addition to defining mechanisms through which H. pylori mediates carcinogenesis, investigations that focus on model organisms colonized by this pathogen may also help to construct a paradigm for other cancers that arise from inflammatory foci within the gastrointestinal tract. More than 80% of hepatocellular carcinomas worldwide are attributable to chronic hepatitis B and hepatitis C infections, and cholangiocarcinoma of the biliary tract is strongly linked to chronic inflammation induced by certain parasites, such as Clonorchis sinensis. Chronic pancreatitis and ulcerative colitis each confer a significantly increased risk of the development of adenocarcinoma within their respective anatomic sites. Thus, a comprehensive understanding of how H. pylori induces gastric disease should facilitate understanding how chronic inflammation leads to pathologic outcomes in other organ systems.
Footnotes
COMPETING INTERESTS
The author declares no competing financial interests.
References
- Amieva M. R., Vogelmann R., Covacci A., Tompkins L. S., Nelson W. J., Falkow S. (2003). Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science 300, 1430–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Chen Y., Reibman J. (2008). Does Helicobacter pylori protect against asthma and allergy? Gut 57, 561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonjakuakul J. K., Canfield D. R., Solnick J. V. (2005). Comparison of Helicobacter pylori virulence gene expression in vitro and in the Rhesus macaque. Infect. Immun. 73, 4895–4904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X., Carlson J., Stoicov C., Li H., Wang T. C., Houghton J. (2005). Helicobacter felis eradication restores normal architecture and inhibits gastric cancer progression in C57BL/6 mice. Gastroenterology 128, 1937–1952 [DOI] [PubMed] [Google Scholar]
- Chen Y., Blaser M. J. (2007). Inverse associations of Helicobacter pylori with asthma and allergy. Arch. Intern. Med. 167, 821–827 [DOI] [PubMed] [Google Scholar]
- Cover T. L., Krishna U. S., Israel D. A., Peek R. M., Jr (2003). Induction of gastric epithelial cell apoptosis by Helicobacter pylori vacuolating cytotoxin. Cancer Res. 63, 951–957 [PubMed] [Google Scholar]
- Eaton K. A., Cover T. L., Tummuru M. K., Blaser M. J., Krakowka S. (1997). Role of vacuolating cytotoxin in gastritis due to Helicobacter pylori in gnotobiotic piglets. Infect. Immun. 65, 3462–3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Omar E. M., Carrington M., Chow W. H., McColl K. E., Bream J. H., Young H. A., Herrera J., Lissowska J., Yuan C. C., Rothman N., et al. (2000). Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 404, 398–402 [DOI] [PubMed] [Google Scholar]
- El-Omar E. M., Rabkin C. S., Gammon M. D., Vaughan T. L., Risch H. A., Schoenberg J. B., Stanford J. L., Mayne S. T., Goedert J., Blot W. J., et al. (2003). Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology 124, 1193–1201 [DOI] [PubMed] [Google Scholar]
- Farrokhi F., Vaezi M. F. (2007). Extra-esophageal manifestations of gastroesophageal reflux. Oral Dis. 13, 349–359 [DOI] [PubMed] [Google Scholar]
- Figueiredo C., Machado J. C., Pharoah P., Seruca R., Sousa S., Carvalho R., Capelinha A. F., Quint W., Caldas C., van Doorn L. J., et al. (2002). Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J. Natl. Cancer Inst. 94, 1680–1687 [DOI] [PubMed] [Google Scholar]
- Fox J. G., Rogers A. B., Ihrig M., Taylor N. S., Whary M. T., Dockray G., Varro A., Wang T. C. (2003a). Helicobacter pylori-associated gastric cancer in INS-GAS mice is gender specific. Cancer Res. 63, 942–950 [PubMed] [Google Scholar]
- Fox J. G., Wang T. C., Rogers A. B., Poutahidis T., Ge Z., Taylor N., Dangler C. A., Israel D. A., Krishna U., Gaus K., et al. (2003b). Host and microbial constituents influence Helicobacter pylori-induced cancer in a murine model of hypergastrinemia. Gastroenterology 124, 1879–1890 [DOI] [PubMed] [Google Scholar]
- Franco A. T., Israel D. A., Washington M. K., Krishna U., Fox J. G., Rogers A. B., Neish A. S., Collier-Hyams L., Perez-Perez G. I., Hatakeyama M., et al. (2005). Activation of β-catenin by carcinogenic Helicobacter pylori. Proc. Natl. Acad. Sci. USA 102, 10646–10651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco A. T., Johnston E., Krishna U., Yamaoka Y., Israel D. A., Nagy T. A., Wroblewski L. E., Piazuelo M. B., Correa P., Peek R. M., Jr (2008). Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res. 68, 379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa A., Shirasaka D., Yamamoto S., Ota H., Yahiro K., Fukada M., Shintani T., Wada A., Aoyama N., Hirayama T., et al. (2003). Mice deficient in protein tyrosine phosphatase receptor type Z are resistant to gastric ulcer induction by VacA of Helicobacter pylori. Nat. Genet. 33, 375–381 [DOI] [PubMed] [Google Scholar]
- Gebert B., Fischer W., Weiss E., Hoffmann R., Haas R. (2003). Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science 301, 1099–1102 [DOI] [PubMed] [Google Scholar]
- Gerhard M., Lehn N., Neumayer N., Boren T., Rad R., Schepp W., Miehlke S., Classen M., Prinz C. (1999). Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc. Natl. Acad. Sci. USA 96, 12778–12783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B. P., Mekalanos J. J. (2002). Rapid genetic analysis of Helicobacter pylori gastric mucosal colonization in suckling mice. Proc. Natl. Acad. Sci. USA 99, 8354–8359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hold G. L., Rabkin C. S., Chow W. H., Smith M. G., Gammon M. D., Risch H. A., Vaughan T. L., McColl K. E., Lissowska J., Zatonski W., et al. (2007). A functional polymorphism of Toll-like receptor 4 gene increases risk of gastric carcinoma and its precursors. Gastroenterology 132, 905–912 [DOI] [PubMed] [Google Scholar]
- Honda S., Fujioka T., Tokieda M., Satoh R., Nishizono A., Nasu M. (1998). Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer Res. 58, 4255–4259 [PubMed] [Google Scholar]
- Ilver D., Arnqvist A., Ogren J., Frick I. M., Kersulyte D., Incecik E. T., Berg D. E., Covacci A., Engstrand L., Boren T. (1998). Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science 279, 373–377 [DOI] [PubMed] [Google Scholar]
- Israel D. A., Salama N., Krishna U., Rieger U. M., Atherton J. C., Falkow S., Peek R. M., Jr (2001a). Helicobacter pylori genetic diversity within the gastric niche of a single human host. Proc. Natl. Acad. Sci. USA 98, 14625–14630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel D. A., Salama N., Arnold C. N., Moss S. F., Ando T., Wirth H. P., Tham K. T., Camorlinga M., Blaser M. J., Falkow S., et al. (2001b). Helicobacter pylori strain-specific differences in genetic content, identified by microarray, influence host inflammatory responses. J. Clin. Invest. 107, 611–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase K., Evers B. M., Hellmich M. R., Guo Y. S., Higashide S., Kim H. J., Townsend C. M., Jr (1997). Regulation of growth of human gastric cancer by gastrin and glycine-extended progastrin. Gastroenterology 113, 782–790 [DOI] [PubMed] [Google Scholar]
- Judd L. M., Alderman B. M., Howlett M., Shulkes A., Dow C., Moverley J., Grail D., Jenkins B. J., Ernst M., Giraud A. S. (2004). Gastric cancer development in mice lacking the SHP2 binding site on the IL-6 family co-receptor gp130. Gastroenterology 126, 196–207 [DOI] [PubMed] [Google Scholar]
- Linz B., Balloux F., Moodley Y., Manica A., Liu H., Roumagnac P., Falush D., Stamer C., Prugnolle F., van der Merwe S. W., et al. (2007). An African origin for the intimate association between humans and Helicobacter pylori. Nature 445, 915–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdavi J., Sonden B., Hurtig M., Olfat F, O., Forsberg L., Roche N., Angstrom J., Larsson T., Teneberg S., Karlsson K. A., et al. (2002). Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science 297, 573–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimuro H., Suzuki T., Nagai S., Rieder G., Suzuki M., Nagai T., Fujita Y., Nagamatsu K., Ishijima N., Koyasu S., et al. (2007). Helicobacter pylori dampens gut epithelial self-renewal by inhibiting apoptosis, a bacterial strategy to enhance colonization of the stomach. Cell Host Microbe 2, 250–263 [DOI] [PubMed] [Google Scholar]
- O’Brien D. P., Israel D. A., Krishna U., Romero-Gallo J., Nedrud J., Medof M. E., Lin F., Redline R., Lublin D. M., Nowicki B. J., et al. (2006). The role of decay-accelerating factor as a receptor for Helicobacter pylori and a mediator of gastric inflammation. J. Biol. Chem. 281, 13317–13323 [DOI] [PubMed] [Google Scholar]
- Ogura K., Maeda S., Nakao M., Watanabe T., Tada M., Kyutoku T., Yoshida H., Shiratori Y., Omata M. (2000). Virulence factors of Helicobacter pylori responsible for gastric diseases in Mongolian gerbil. J. Exp. Med. 192, 1601–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi N., Yuasa H., Tanaka S., Sawa H., Miura M., Matsui A., Higashi H., Musashi M., Iwabuchi K., Suzuki M., et al. (2008). Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc. Natl. Acad. Sci. USA 105, 1003–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek R. M., Jr, Blaser M. J. (2002). Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer 2, 28–37 [DOI] [PubMed] [Google Scholar]
- Peek R. M., Jr, Crabtree J. E. (2006). Helicobacter infection and gastric neoplasia. J. Pathol. 208, 233–248 [DOI] [PubMed] [Google Scholar]
- Peek R. M., Wirth H. P., Moss S. F., Yang M., Abdalla A. M., Tham K. T., Zhang T., Tang L. H., Modlin I. M., Blaser M. J. (2000). Helicobacter pylori alters gastric epithelial cell cycle events and gastrin secretion in Mongolian gerbils. Gastroenterology 118, 48–59 [DOI] [PubMed] [Google Scholar]
- Philpott D. J., Belaid D., Troubadour P., Thiberge J. M., Tankovic J., Labigne A., Ferrero R. L. (2002). Reduced activation of inflammatory responses in host cells by mouse-adapted Helicobacter pylori isolates. Cell Microbiol. 4, 285–296 [DOI] [PubMed] [Google Scholar]
- Rhead J. L., Letley D. P., Mohammadi M., Hussein N., Mohagheghi M. A., Eshagh Hosseini M., Atherton J. C. (2007). A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology 133, 926–936 [DOI] [PubMed] [Google Scholar]
- Rieder G., Merchant J. L., Haas R. (2005). Helicobacter pylori cag-type IV secretion system facilitates corpus colonization to induce precancerous conditions in Mongolian gerbils. Gastroenterology 128, 1229–1242 [DOI] [PubMed] [Google Scholar]
- Salama N. R., Otto G., Tompkins L., Falkow S. (2001). Vacuolating cytotoxin of Helicobacter pylori plays a role during colonization in a mouse model of infection. Infect. Immun. 69, 730–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solnick J. V., Hansen L. M., Salama N. R., Boonjakuakul J. K., Syvanen M. (2004). Modification of Helicobacter pylori outer membrane protein expression during experimental infection of rhesus macaques. Proc. Natl. Acad. Sci. USA 101, 2106–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozzi M., Crosatti M., Kim S. K., Romero J., Blaser M. J. (2001). Heterogeneity of Helicobacter pylori cag genotypes in experimentally infected mice. FEMS Microbiol. Lett. 203, 109–114 [DOI] [PubMed] [Google Scholar]
- Tebbutt N. C., Giraud A. S., Inglese M., Jenkins B., Waring P., Clay F. J., Malki S., Alderman B. M., Grail D., Hollande F., et al. (2002). Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat. Med. 8, 1089–1097 [DOI] [PubMed] [Google Scholar]
- Wang T. C., Dangler C. A., Chen D., Goldenring J. R., Koh T., Raychowdhury R., Coffey R. J., Ito S., Varro A., Dockray G. J., et al. (2000). Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology 118, 36–47 [DOI] [PubMed] [Google Scholar]
- Watanabe T., Tada M., Nagai H., Sasaki S., Nakao M. (1998). Helicobacter pylori infection induces gastric cancer in Mongolian gerbils. Gastroenterology 115, 642–648 [DOI] [PubMed] [Google Scholar]
- Wirth H. P., Beins M. H., Yang M., Tham K. T., Blaser M. J. (1998). Experimental infection of Mongolian gerbils with wild-type and mutant Helicobacter pylori strains. Infect. Immun. 66, 4856–4866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong B. C., Lam S. K., Wong W. M., Chen J. S., Zheng T. T., Feng R. E., Lai K. C., Hu W. H., Yuen S. T., Leung S. Y., et al. (2004). Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 291, 187–194 [DOI] [PubMed] [Google Scholar]
- Zheng Q., Chen X. Y., Shi Y., Xiao S. D. (2004). Development of gastric adenocarcinoma in Mongolian gerbils after long-term infection with Helicobacter pylori. J. Gastroenterol. Hepatol. 19, 1192–1198 [DOI] [PubMed] [Google Scholar]