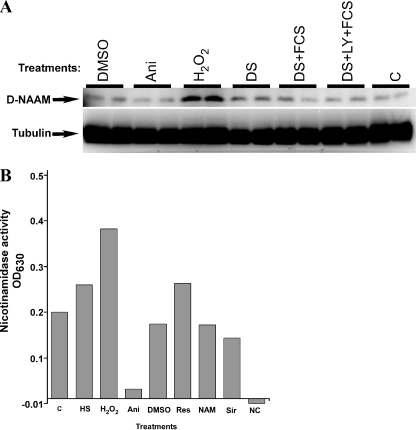
FIGURE 3.
Oxidative stress enhances D-NAAM protein expression and activity. A, Drosophila S2 cells were cultured under the indicted growth condition (as in Fig. 2A) for 18 h, and total cell lysates were analyzed for D-NAAM protein expression using a carboxyl-terminal D-NAAM peptide antibody (top panel). Tubulin immunoblotting was used to confirm protein loading (bottom panel). Cell treatments and the protein expression analysis were performed in duplicate. DMSO, dimethyl sulfoxide. B, D-NAAM was immunopurified from S2 cells cultured under the indicated conditions using the carboxyl-terminal D-NAAM antibody and assayed for nicotinamidase activity as in Fig. 1. NC, negative control, no substrate (NAM) was added in the nicotinamidase assay. The experiment was performed in duplicate and is representative of three independent experiments. The treatments were as follows: C, control; HS, heat shock, 2 h at 37 °C; H2O2, 15 μm, 18 h; Ani, 10 μg/ml anisomycin, 18 h; DMSO, 0.1% Me2SO, 18 h (control); Res, 100 μm resveratrol, 18 h; NAM, 40 mm nicotinamide, 18 h; Sir, 50 μm sirtinol, 18 h.