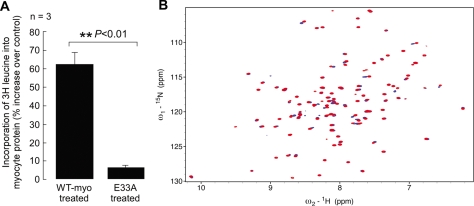
FIGURE 6.
Effect of E33A mutant myotrophin on increase in myocyte protein synthesis and structural comparison of wild type and E33A mutant protein. A, incorporation of 3[H]leucine into neonatal rat ventricular myocytes showed significantly (p < 0.05) less increase in protein synthesis in E33A-treated cells compared with wild type (WT) myotrophin-treated cells. The data show the means (± S.E.) of three independent sets of results. B, 15N/1H heteronuclear single quantum coherence spectra collected from 15N-labeled wild type and E33A myotrophin. The spectrum of wild type myotrophin is shown in blue, overlaid with the E33A spectrum in red. The spectra of the two proteins are mostly identical or very similar. The well dispersed spectrum of the E33A mutant shows that the mutation does not disrupt the overall folding of the protein or cause global changes to the protein fold but rather only limited local changes.