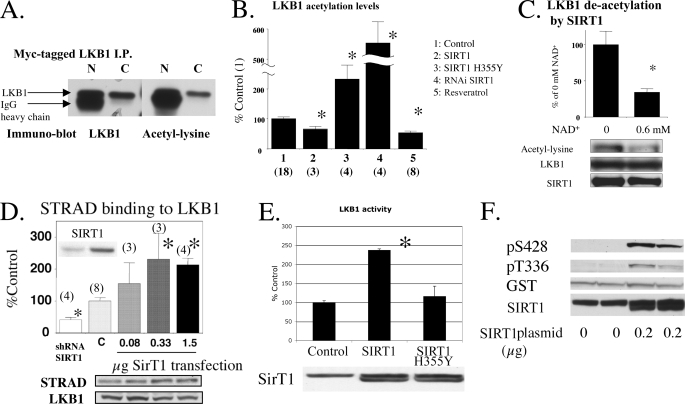
FIGURE 1.
LKB1 acetylation and the effects of SIRT1. A, lysine acetylation of an exogenously expressed LKB1. Myc-tagged LKB1 expressed in HEK293T cells was immunoprecipitated with native (N) and covalently linked (C) anti-Myc antibody-protein A beads. B, modulation of LKB1 acetylation by SIRT1 in 293T cells. Plasmids expressing GST-LKB1 were transfected into HEK293T cells together with pcDNA or shRNA lentivirus for control, SIRT1 WT, or H355Y plasmid (0.5 μg) or shRNA for SIRT1 lentivirus. In addition, some cells were treated with 50–100 μm resveratrol for 0.5–1 h. The cells were harvested for Western blotting to quantify LKB1 acetylation. *, p <0.05 versus control. Number of samples are shown in parentheses. C, direct deacetylation of GST-LKB1 by SIRT1 in vitro. Purified GST-LKB1 was incubated in a SIRT1 reaction buffer containing 1 μl of recombinant SIRT1 solution with/without 0.6 mm NAD+ at 30 °C for 30 min (n = 6). D, SIRT1 expression levels affect STRAD association with LKB1. The 293T cells were transfected with SIRT1 plasmid or shRNA for sirt1. STRAD binding to LKB1 was assessed by calculating the STRAD to LKB1 ratio and is expressed as percent of the ratio in control cells (*, p < 0.05 versus C. Number of samples are in parenthesis). E and F, effects of SIRT1 overexpression on LKB1 activity (E) and phosphorylation (F). E, 293T cells were transfected with the 0.2 μg of SIRT1 plasmid. LKB1 activity was assessed in LKB1-immunoprecipitated samples (*, p < 0.005 versus control (pcDNA) plasmid, n = 3). The H355Y mutant did not increase LKB1 activity. F, 293T cells were co-transfected with 0.2 μg of SIRT1 and 0.5 μg of GSTl-LKB1 plasmids and blotted with the indicated antibodies.