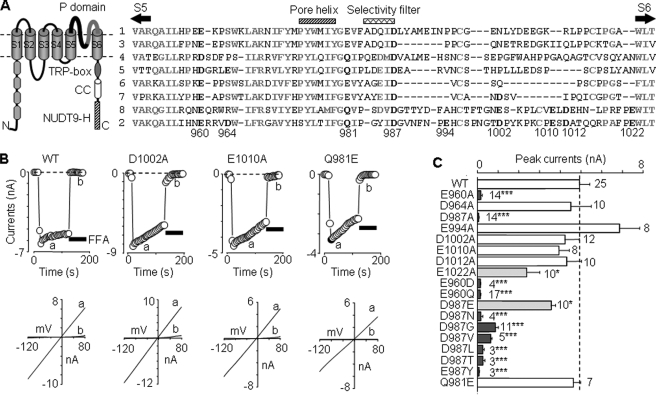
FIGURE 1.
Effects of mutating pore residues on functional channel expression. A, left, schematic presentation of membrane topology of the TRPM2 subunit. It contains six transmembrane segments (S1–S6) with a pore loop between S5 and S6, four TRPM homology domains in the N terminus (N), and a stretch of highly conserved residues (TRP-box), a coiled-coil (CC) domain and NUTD9 homology (NUTD9-H) domain in the C terminus (C). Right, amino acid sequence alignment of human TRPM1–8 pore loops using ClustalW. The highly conserved residues are highlighted in bold and gray, and the residues in TRPM2 examined in this study (numbering according to hTRPM2) and those conserved in other TRPM subunits are in bold and black. B, representative ADPR-evoked currents at –80 mV (top, denoted by circles) and I/V curves (bottom), obtained by voltage ramps applied every 5 s, from cells expressing the WT or indicated mutant channels. The horizontal bars here and in the other figures represent application of flufenamic acid (0.5 mm; black bars) or anthranilic acid (20 μm; hatched bars). C, summary of the ADPR-evoked peak currents in cells expressing the WT or indicated mutant channels. The dotted line shows the mean current for the WT channel. The number of cells examined in each case is indicated. *, p < 0.05, and ***, p < 0.001, when compared with WT.