Table 1.
Nucleophile and electrophile scope in acyl aminal synthesis.a
| entry | substrate | solvent | electrophile | nucleophile | major product | yield (dr)b |
|---|---|---|---|---|---|---|
| 1 |
10 |
CH2Cl2 | iPrC(O)Cl | MeOH |
11 |
75% (2:3:1) |
| 2 |
10 |
THF | iPrC(O)Cl | MeOH |
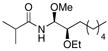
12 |
64% (1:4:1) |
| 3c |
10 |
CH2Cl2Mg(ClO4)2 | iPrC(O)Cl | MeOH |
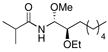
11 |
71% (5:7:1) |
| 4 |
10 |
CH2Cl2 | MeOCH2C(O)Cl | MeOH |

13 |
69% (1:7:1) |
| 5 |
10 |
CH2Cl2 | CbzCl | MeOH |
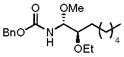
14 |
64% (1:5:1) |
| 6 |
10 |
CH2Cl2 | Ms2O | MeOH |
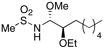
15 |
24% (2:4:1) |
| 7 |
10 |
CH2Cl2 | iPrC(O)Cl | tBuOH |
16 |
71% (2:0:1) |
| 8 |
10 |
CH2Cl2 | iPrC(O)Cl | PhOH |
17 |
69% (5:6:1) |
| 9 |
10 |
CH2Cl2 | iPrC(O)Cl | PhSH |
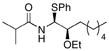
18 |
72% (7:0:1) |
Representative procedure: Cp2Zr(H)Cl (1.2 equiv) was added to a solution of the substrate in the solvent (0.1 M). The mixture stirred for 10 min at rt, then was cooled to 0 °C. The electrophile (1.2–1.5 equiv) was added and stirred for 10 min. The nucleophile (20 equiv) was added and the reaction was stirred for a few additional minutes.
Yields refer to the sum of the yields of the diastereomers.
Nucleophilic addition was conducted at −78 °C.
