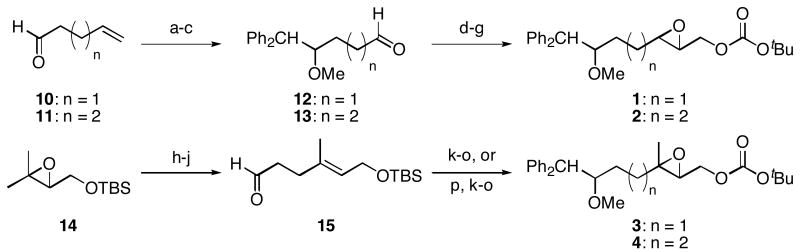
Scheme 2.
Synthesis of monoepoxide substrates.
Reagents and conditions a) Ph2CH2, nBuLi, THF, 0 °C, 83% (n = 1), 70% (n = 2). b) NaH, DMF, 0 °C, then MeI, 97% (n = 1), 95% (n = 2). c) O3, CH2Cl2, −78 °C, then Ph3P, rt, 96% (n = 1 or 2). d) (EtO)2P(O)CH2CO2Et, NaH, THF, 0 °C, 94% (n = 1), 93% (n = 2). e) DIBAL-H, THF, −78 °C. f) m-CPBA, NaHCO3, CH2Cl2, 0 °C 70% (n = 1), 91% (n = 2), two steps. g) Boc2O, N-methylimidazole, PhMe, 0 °C, 90% (n = 1), 84% (n = 2). h) 2,2,6,6-Tetramethylpiperidine, nBuLi, Et2AlCl, benzene, 0 °C, 90%. i) (EtO)3CCH3, propionic acid, 145 °C, 96%. j) DIBAL-H, CH2Cl2, −78°C. k) Ph2CH2, nBuLi, THF, 0 °C, 70% (n = 1), 79% (n = 2). l) NaH, DMF, 0 °C, then MeI. m) Bu4NF, THF, 100% (n = 1), 97% (n = 2), two steps. n) m-CPBA, NaHCO3, CH2Cl2, 0 °C, 95% (n = 1), 99% (n = 2). o) Boc2O, N-methylimidazole, PhMe, 0 °C, 86% (n = 1), 89% (n = 2). p) Ph3P+CH2OMe Cl-, NaHMDS, THF, −78 °C, then Hg(OAc)2, THF, H2O, KI, 82%.