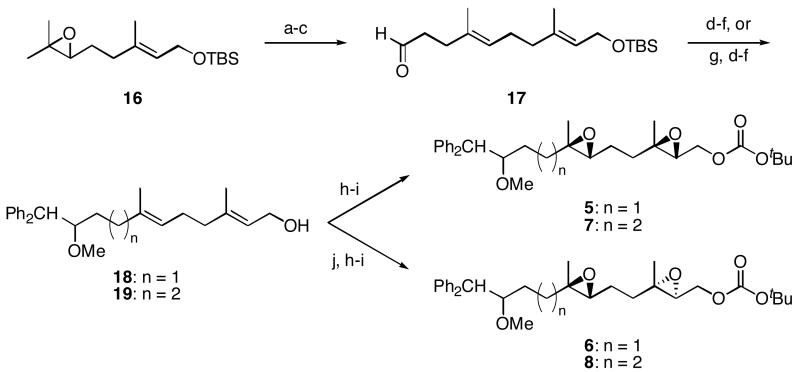
Scheme 3.
Synthesis of diepoxide cyclization substrates 5-8.
Reagents and conditions a) 2,2,6,6-Tetramethylpiperidine, nBuLi, Et2AlCl, benzene, 0 °C, 92%. b) (EtO)3CCH3, propionic acid, 145 °C, 94%. c) DIBAL-H, CH2Cl2, −78°C, 92%. d) Ph2CH2, nBuLi, THF, 0 °C, 80% (n = 1), 80% (n = 2). e) NaH, DMF, 0 °C, then MeI. f) Bu4NF, THF, 97% (n = 1), 98% (n = 2), two steps. g) Ph3P+CH2OMe Cl-, NaHMDS, THF, −78 °C, then Hg(OAc)2, THF, H2O, KI, 91%. h) Shi catalyst, KHSO5, (MeO)2CH2, CH3CN, H2O, 0 °C, 88% (5), 91% (6), 64% (7). i) Boc2O, N-methylimidazole, PhMe, 0 °C, 93% (5), 86% (6), 78% (7), 82% (8, two steps). j) (+)–Diisopropyl tartrate, tBuOOH, Ti(OiPr)4, CH2Cl2, −25 °C, 96% (n = 1), 97% (n = 2).