Figure 2.
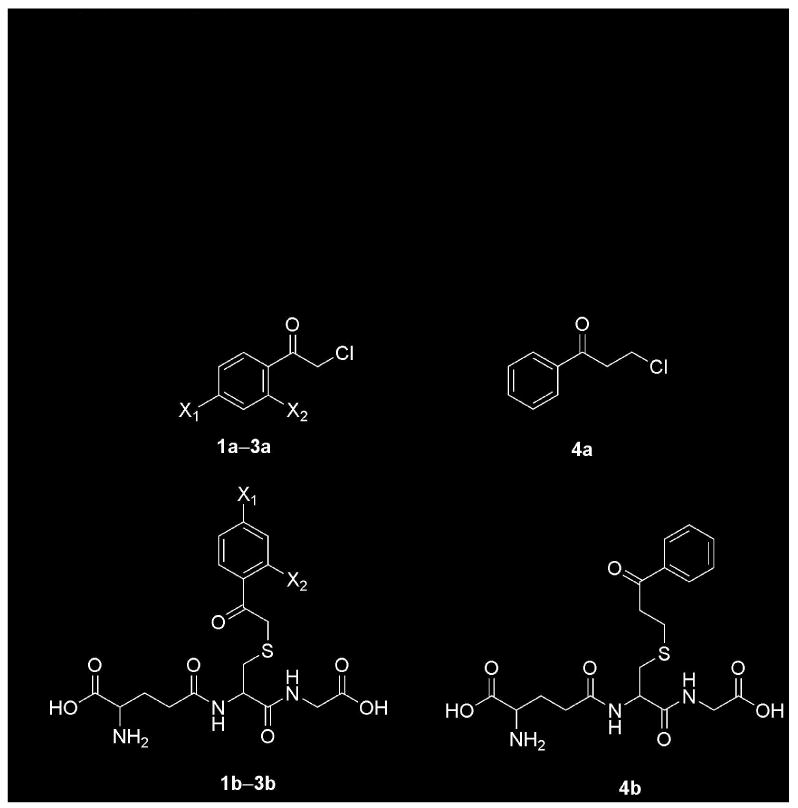
Structures of precursor α- and β-haloketones, S-(phenacyl)glutathiones, and S-(2-benzoylethyl)glutathione. 1a, 2-chloroacetophenone, X1 = X2 = H; 1b, S-(phenacyl)glutathione, X1 = X2 = H; 2a, 2,2′4′-trichloroacetophenone, X1 = X2 = Cl; 2b, S-(2′,4′-dichlorophenacyl)glutathione, X1 = X2 = Cl; 3a, 2-chloro-4′-fluoroacetophenone, X1 = F, X2 = H; 3b, S-(4′-fluorophenacyl)glutathione, X1 = F, X2 = H; 4a, 3-chloropropiophenone; 4b, S-(2-benzoylethyl)glutathione.
