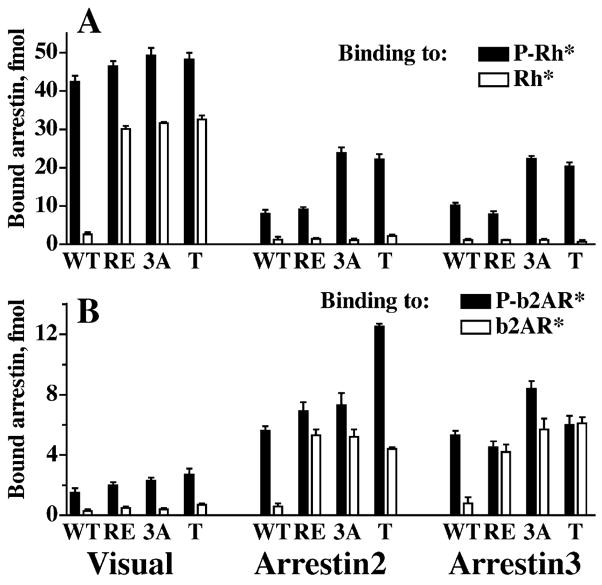
Fig. 11.
Phosphate-dependent activation mechanism is conserved in the arrestin family. Comparative binding of WT and mutant forms of visual, arrestin2, and arrestin3 to phosphorylated and unphosphorylated light-activated rhodopsin (A) and b2AR (B). The mutations destabilizing the polar core and 3-element interaction produce a similar phenotype in all 3 arrestins: enhanced binding to the unphosphorylated active cognate and to the phosphorylated active non-cognate receptor, but not to the unphosphorylated active non-cognate receptor. The mutations in visual, arrestin2 and 3, respectively, are designated, as follows: RE: R175E, R169E, and R170E; 3A, triple alanine substitution in the C-tail: FVF→AAA (375–377), IVF→AAA (386–388), and IVF→AAA (386–388); T, C-terminal truncation yielding 1–378, 1–382, and 1–392 mutants.