Fig. 16.
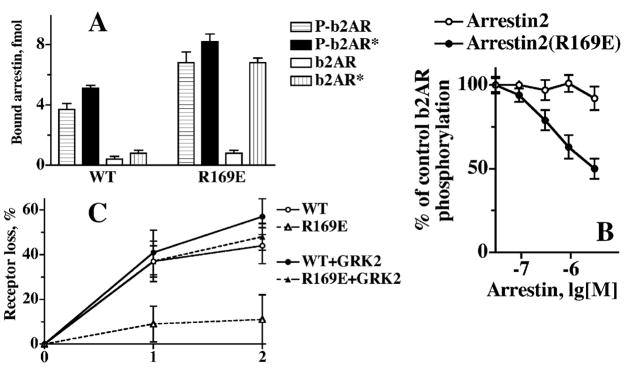
The formation of a transient arrestin–receptor complex prevents receptor down-regulation. In contrast to WT arrestin2, R169E mutant binds with high affinity to unphosphorylated b2AR* (A), thereby competing with GRK2 and inhibiting receptor phosphorylation (B). The expression of the R169E mutant in HEK cells at levels sufficiently high to out-compete GRKs and endogenous arrestins and to form complexes with unphosphorylated b2AR* prevents the loss of the receptor even after very long agonist exposure, in contrast to the overexpression of WT arrestin2 (C). However, concomitant overexpression of GRK2 (that ensures that the receptor is phosphorylated faster, so that both WT and R169E arrestins bind phosphorylated receptor) “rescues” receptor down-regulation (C). The R169E mutant facilitates receptor cycling in two ways. First, deactivation of P-b2AR (due to agonist loss in the endosome) does not dramatically reduce arrestin binding, whereas deactivation of unphosphorylated b2AR sharply reduces arrestin binding, so that upon internalization the mutant dissociates from the receptor faster. Second, after the release of WT arrestin the emerging multi-phosphorylated receptor must be fully dephosphorylated before it can be recycled, whereas the dissociation of R169E immediately releases the unphosphorylated recycling-competent receptor. Thus, the decrease in stability of the arrestin–receptor complex facilitates receptor cycling, and rapid recycling, in its turn, prevents receptor down-regulation (Pan et al., 2003).