Fig. 3.
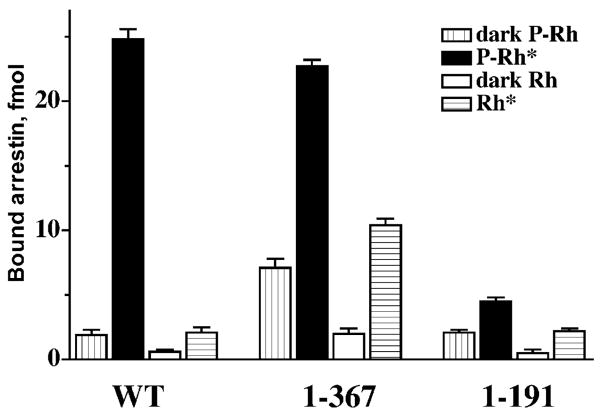
The function of the arrestin C-domain and C-tail. The deletion of the arrestin C-tail yields truncated arrestin(1–367) that binds P-Rh* essentially as well as full-length (WT) arrestin. This deletion dramatically decreases arrestin selectivity for P-Rh*, enhancing the binding to dark P-Rh and unpho-sphorylated Rh*, suggesting that the C-tail is a regulatory element “suppressing” the interactions with non-preferred forms of rhodopsin. The deletion of the whole C-terminal half of the molecule yields arrestin(1–191). This “mini-arrestin” demonstrates essentially the same binding to dark P-Rh and unphosphorylated Rh* as WT arrestin, but its binding to P-Rh* is many times lower. In the case of this mutant (in sharp contrast to WT arrestin), P-Rh* binding roughly equals the sum of the binding to dark P-Rh and Rh*. Thus, arrestin(1–191) does not have an additional binding site that can be mobilized to ensure arrestin selectivity (Gurevich & Benovic, 1992).