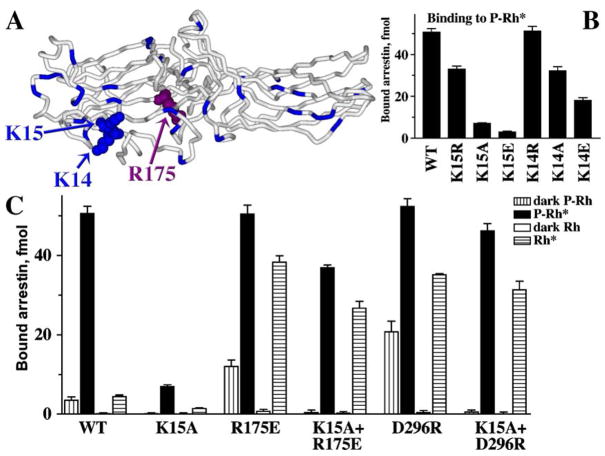
Fig. 6.
How do the phosphates get to the shielded Arg175? (A) In the basal conformation arrestin’s main phosphate sensor Arg175 (highlighted with the atoms shown) in the polar core is shielded, whereas numerous other positively charged residues in the N-domain (highlighted) are highly exposed. (B) Lysines 14 and15 (highlighted with the atoms shown in panel A) in β-strand I interact with receptor-attached phosphates, as evidenced by the progressive decrease of P-Rh* binding with neutralization and reversal of their charges. (C) The K15A mutation dramatically reduces WT arrestin binding to P-Rh*. However, in the context of arrestin mutants in which the polar core is already disrupted (R175E or D296R), the effects of the same K15A mutation on P-Rh* binding are mild (K15A+R175E and K15A+D296R). Importantly, the K15A mutation suppresses arrestin binding to dark P-Rh (which is mediated solely by phosphate interactions) in any context, supporting the identification of Lys15 as one of the residues directly binding phosphates. Thus, the presence of Lys15 is required for arrestin binding to P-Rh* only when the polar core is intact, suggesting that its function is to “meet” the phosphates first and then “guide” them to the polar core (adapted from Vishnivetskiy et al., 2000).