Fig. 8.
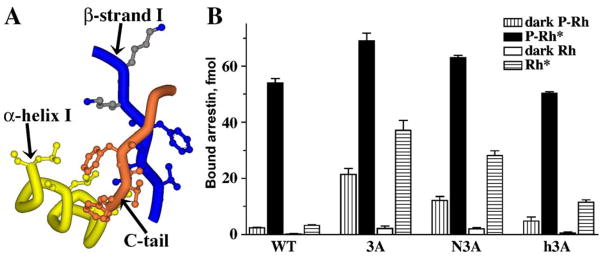
The 3-element interaction: another “clasp” holding arrestin in its basal state. (A) The 3-element interaction between β-strand I and α-helix I of the N-domain and β-strand XX of the C-tail involves triplets of bulky hydrophobic residues in each element (Val11+Ile12+Phe13, Leu103+Leu107+Leu111, and Phe375+Val376+Phe377, respectively). Disrupting the 3-element interaction by replacing the hydrophobic residues with alanines in β-strand XX (3A), β-strand I (N3A), or α-helix I (h3A) yields constitutively active mutants, suggesting that it is disrupted in WT arrestin by P-Rh*. Two highly conserved lysines (Lys14 and Lys15) are present immediately downstream of the participating hydrophobic residues in β-strand I. The movements accompanying phosphate binding to Lys15 and Lys14 likely melt the short β-strand I, disrupting the hydrophobic interaction of adjacent residues with the arrestin C-tail and α-helix (adapted from Vishnivetskiy et al., 2000).