Summary
The majority of women who quit smoking during pregnancy will resume smoking during the postpartum period. Little is known, however, about the predictors of postpartum relapses to smoking. Changes in mood and increases in concerns about weight are common during the postpartum period, and these factors may affect women's postpartum smoking behavior. In this paper, we present a model of the relationship among mood, weight concerns and postpartum smoking. Data from previous postpartum relapse prevention trials are reviewed and evidence of a connection between changes in mood and weight concerns to postpartum relapse is presented. Directions for future research on the prevention of smoking relapses during the postpartum period, and the roles of mood and weight concerns in smoking relapse are presented.
Keywords: Postpartum, smoking, relapse prevention, mood, weight
Introduction
Twenty-two percent of American women smoke (U.S. Department of Health and Human Services, 2001), and information that can help decrease the rate of smoking among women is important to the public health. In recent decades, the prevalence of smoking among pregnant women has decreased (Ebrahim et al., 2000; Mathews, 2001) and numerous studies have documented the success of prenatal smoking cessation interventions (e.g., Ershoff et al., 1989; Floyd et al., 1993). However, despite the decline in prenatal smoking rates, the majority of women who quit during pregnancy will resume smoking during the year following childbirth (Fingerhut et al., 1990).
Approximately 25% of women who quit smoking during pregnancy will relapse within one month of delivery. By four months postpartum, approximately 43% to 52% of women will have resumed smoking, and 60% to 70% of women will return to smoking by six months postpartum (Fingerhut et al., 1990; McBride and Pirie, 1990; Mullen et al., 1990; Ratner et al., 2000). Because many women quit smoking during pregnancy and subsequently relapse, an increased understanding of the factors related to smoking during the postpartum period may be particularly helpful in improving women's smoking cessation efforts. Investigators, therefore, have begun to consider issues related to the prevention of postpartum smoking relapse.
However, despite considerable research on the relationships of mood and weight concerns to women's smoking behavior in general, little is known about the role mood or weight concerns might play in smoking relapse during the postpartum period. In this paper, we present a model of the relationship among changes in mood, concerns about weight and postpartum smoking. This paper is not intended to serve as a comprehensive review of the risk factors associated with postpartum smoking. Rather, we provide a selective review of studies related to our hypothesis that mood and weight concerns may relate to smoking during the postpartum period. Specifically, after briefly reviewing the correlates of postpartum smoking, we examine the evidence that mood and weight concerns relate to postpartum smoking relapse and present suggestions for future research.
The importance of preventing postpartum smoking
There are several reasons to target postpartum smoking relapse. First, women who resume smoking after childbirth expose their young children to tobacco smoke, which has been linked to a multitude of problems. Exposure to tobacco smoke has been related to sudden infant death syndrome, ear infections, respiratory illness and asthma (Dybing and Sanner, 1999; Ey et al., 1995). Environmental tobacco smoke also has been related to deficits in cognitive and behavioral performance among children (Cornelius and Day, 2000; Kahn et al., 2002; Maughan et al., 2001).
Second, postpartum smoking places a mother at risk for the deleterious health risks of smoking. Although smoking is associated with numerous negative health consequences for individuals of both genders, it presents a greater health risk for women than for men. For example, after controlling for other risk factors, women, when compared to men, are at greater risk for lung cancer (Kure et al., 1996; Zang and Wynder, 1996), bladder cancer (Castelao et al., 2001), respiratory symptoms (Langhammer et al., 2000) and myocardial infarction (Prescott et al., 1998) as a result of smoking. Women also suffer additional risks from smoking, including cervical cancer (Castle et al., 2002), reproductive complications and menstrual dysfunction (Baron et al., 1990) that do not affect men.
Finally, there are several unique aspects of the postpartum period that may increase the likelihood that efforts to encourage women to maintain smoking abstinence at this time will be effective. Many women who quit during pregnancy will spend up to eight months smoke-free. These women, presumably, have overcome the acute nicotine withdrawal symptoms, broken many habitual associations to smoking and developed some successful strategies for coping with urges to smoke by the end of pregnancy. Moreover, the adoption of her new role as the caretaker for a newborn may itself increase a woman's motivation to stay quit. Indeed, most women report a desire to remain abstinent after delivery. Secker-Walker and colleagues (1995) asked women about their intention to avoid cigarettes after the baby's birth using a five-point Likert scale, and 96% of the women reported that they were highly motivated to stay quit. Despite the many reasons to remain abstinent, however, most women relapse to smoking. Thus, efforts to prevent postpartum smoking have intensified in the past decade.
Postpartum relapse prevention
Several studies have examined postpartum smoking relapse prevention. MEDLINE and PsycINFO were searched using the key words “postpartum”, “relapse” and “smoking” or “tobacco”. This search generated 47 published articles. Review or survey articles, and qualitative reports were excluded and the remaining 39 studies were further evaluated. Studies in which a clinical trial was conducted and data on postpartum smoking were collected were included in the review and are listed in Table 1.
Table 1.
Postpartum relapse prevention trials
| Study | Participants (sample size)a |
Design | Intervention components |
Intervention providers |
Follow-up | Abstinence rates |
Correlates of abstinence |
Smoking assessment |
|---|---|---|---|---|---|---|---|---|
| Peterson et al. (1992) | smokers recruited from prenatal clinics at large HMO (N=224) | randomized clinics to intervention 1 (I1) or intervention 2 (I2) |
I1: manual and audiotape with section on pp maintenance I2: manual and tape + staff training + letters signed by physician mailed in 8th month of pg and 1st month pp |
I2: obstetricians and nursing staff trained on effects of smoking; no counseling | 8 wks | C=57.1% I1=61.3% I2=79.3% (p=0.05 for C vs. I2) |
none reported | no |
| Secker-Walker et al. (1995) | self-reported nonsmokers recruited at first prenatal visit (N=175) | randomized to UC or I | individual counseling about relapse prevention during pregnancy. Final visit (36 wks) addressed pp period. | trained counselors, booklet | 8–54 mos | I=33% C=32% (NS) |
none reported | self-reportb |
| Wall et al. (1995) | self-reported smoking within one month of pregnancy at first pediatric visit (N=858) | randomized practices to minimal (I1) or extended intervention (I2) |
I1: packet of written materials after delivery I2: packet and interventions during first four well-baby visits |
trained pediatricians | 6 mos | I1=37% I2=47% (p<0.01) |
partner smoking, less education associated with relapse | self-report |
| Gielen et al. (1997) | self-reported smoking before 28 weeks of pregnancy (N=98) | randomized to I or UC | written guide and brief counseling about relapse prevention | trained peer health counselor | 6 mos | I=15% C=4%d (not compared) |
none reported | cotinine |
| Severson et al. (1997) | self-reported smoking within one month of pregnancy, regardless of current smoking status (N=1026) | randomized practices to minimal (I1) or extended intervention (I2) |
I1: packet of written materials after delivery I2: packet and interventions during first four well-baby visits |
trained pediatricians, nurses & physician assistants | 12 mos | I1=26% I2=33% (NS) |
partner smoking, less confidence to remain quit, alcohol use, less weight lossc associated with relapse | self report |
| Secker-Walker et al. (1998) | self-reported nonsmokers recruited at first prenatal visit (N=125) | randomized to UC or I | individual counseling about relapse prevention during pregnancy. Final visit (36 wks) addressed pp period. | trained nurses | 12 mos | I=45% C=52% (NS) |
none reported | self-reportb |
| McBride et al. (1999) | smokers recruited at first prenatal visit at large HMO (N=897) | randomized to one of three intervention groups (I1, I2, I3) |
I1: booklet only I2: booklet and prenatal calls I3: booklet, prenatal calls and calls, mailings pp |
mailings and telephone | 8 wks: | I1=56% vs I2=65% I3=67% (p=0.09) |
none reported | cotinine |
| 6 mos: | I1=45%, I2=47% vs. I3=57% (p=0.09) |
|||||||
| 12 mos: | I1=42%, I2=42%, I3=43% (NS) |
|||||||
| Johnson et al. (2000) | self reported quitters recruited after delivery (N=251) | randomized to UC or I | one individual appointment in the hospital and follow-up telephone callse | trained nurses | 6 mos | I=38% C=27% (NS) |
high mental healthf, self efficacy for negative affect associated with decreased likelihood of daily smoking | CO or self-reporth |
| Ratner et al. (2000)g | self reported quitters recruited after delivery (N=238) | randomized to UC or I | one individual appointment in the hospital and follow-up telephone callse | trained nurses | 12 mos | I=21% C=19% (NS) |
breast feeding, high mental healthf associated with decreased likelihood of daily smoking | CO or self-reporth |
| Van't Hof et al. (2000) | self reported quitters recruited after delivery (N=287) | randomized to UC or I | one individual appointment | nurses, and trained pediatricians | 6 mos | I=42% C=38% (NS) |
confidence to stay quit, social encouragement and few smoking friends/family 0 associated with abstinence | self-report |
| Valanis et al. (2001) | smokers recruited from large HMO at first prenatal appt. (N=2055) | quasi-experimental | videos, letters or self-help brochures; groups compared before during and after staff training | hospital nurses, lactation consultants; pediatric staff | 12 mos | I: 14% C:11% (p=0.04) |
quitting before first prenatal, older maternal age associated with abstinence | self-report |
Note. HMO=Health Maintenance Organization; I=Intervention or Treatment Group; mos=months; NS=No significant difference between groups; pg=pregnancy; pp=postpartum; UC=Usual Care or Control Group.
Sample size reflects the size used in analysis of postpartum relapse.
Both studies by Secker-Walker et al. focused on preventing relapse to smoking during pregnancy and used urinary cotinine during pregnancy to determine abstinence. Women who had relapsed during pg were also followed pp.
Weight loss interacted with treatment condition and relapsing was associated with having lost more weight for mothers in the extended intervention condition (I2).
107 of 467 subjects enrolled in the trial provided data on postpartum smoking of whom only 98 were randomized to intervention or UC. Thus, there was insufficient power to evaluate the postpartum intervention.
Only 25% of the participants received all of the eight planned phone calls.
Used a five item measure of mental health.
Follow up to the Johnson et al. (2000) study.
Used self report to define smoking status when CO was not collected.
As summarized in Table 1, information about relapse prevention has been presented during obstetrical visits (Gielen et al., 1997; McBride et al., 1999; Secker-Walker et al., 1995; 1998), in the hospital following delivery (Johnson et al., 2000), and during pediatrician visits (Wall et al., 1995; Severson et al., 1997; Van't Hof et al., 2000). The interventions have involved selfhelp booklets and videos presented prior to delivery (McBride et al., 1999) or in pediatric offices (Severson et al., 1997; Van't Hof et al., 2000), advice presented during routine prenatal visits (Secker-Walker et al., 1995; 1998), and relapse prevention counseling delivered by a nurse (Johnson et al., 2000; Ratner et al., 2000; Van't Hof et al., 2000) or a peer counselor trained for the study (Gielen et al., 1997).
In general, postpartum relapse prevention studies have found little difference in the rates of postpartum smoking between women who did and did not receive an intervention. Although some postpartum relapse prevention interventions have been successful in increasing the amount of time women remain abstinent after delivery (McBride et al., 1999; Ratner et al., 2000; Severson et al., 1997), the long-term efficacy of these interventions has been equivocal. For example, Secker-Walker and colleagues (1998) compared the relative efficacy of physician advice about remaining quit delivered during prenatal visits and physician advice plus referral to an on-site relapse prevention counselor in helping mothers stay quit. Women in the intervention group received five individual prenatal counseling sessions during which they discussed ways to resist urges to smoke and received advice on the importance of not smoking after childbirth. At one year postpartum, there was no difference in the rates of relapse between women who had received the relapse prevention counseling and those who had not.
Similarly, in another study, women in both intervention and control groups reported comparable levels of intent to remain quit after delivery, which suggests that a prenatal intervention did not increase women's motivation to maintain postpartum abstinence (Secker-Walker et al., 1995). Moreover, some of the studies (Secker-Walker et al., 1995; 1998; Valanis et al., 2001) focused on preventing relapse during pregnancy among women who had quit in the first trimester but include a followup assessment during the postpartum period. Thus there are only a few studies in which the prevention of postpartum smoking relapse is the focus of the trial.
Methodologic problems also have limited the utility of available research. Many of the studies summarized in Table 1 did not provide biochemical verification of smoking status during the postpartum interval. In addition to being part of the current standard recommendations for the assessment of smoking cessation (SRNT Subcommittee on Biochemical Verification, 2002), biochemical verification is important among new mothers who, like pregnant women (Campbell et al., 2001; Owen and McNeill, 2001), may be prone to under-report smoking. Moreover, few studies have provided a thorough examination of the possible correlates of postpartum relapse. In summary, prenatal relapse prevention interventions appear to be unsuccessful in increasing the percentage of women maintaining postpartum abstinence. Further, additional research on the correlates of postpartum relapse and the efficacy of relapse prevention programs delivered during this period may provide critical information to guide future intervention efforts.
Factors related to postpartum relapse
Not surprisingly, several of the factors that are associated with smoking relapses in general also have been related to postpartum smoking. The use of alcohol (Severson et al., 1995), membership in a minority group (Carmichael et al., 2000) and higher levels of nicotine dependence (Carmichael et al., 2000; McBride et al., 1992; Ratner et al., 2000) all have been associated with a resumption of smoking after pregnancy. In addition to these general factors, investigators have found some variables that are conceptually linked to pregnancy and the postpartum period to be associated with relapse risk.
One factor, having a partner who smokes, has received consistent support as a correlate of postpartum smoking (McBride and Pirie, 1990; McBride et al., 1992; Ratner et al., 2000; Severson et al., 1995; 1997; Stotts et al., 2000; Wall et al., 1995). Similarly, a lack of intention to remain quit after delivery (Mullen et al., 1997) has been related to postpartum relapse. In contrast, support for another frequently hypothesized risk factor, bottle rather than breast feeding the baby, has been inconsistent. Although some studies have found bottle feeding (McBride and Pirie, 1990; Ratner et al., 2000) to predict smoking relapse, others (Severson et al., 1997; Stotts et al., 2000) have not observed a relationship between feeding method and postpartum smoking.
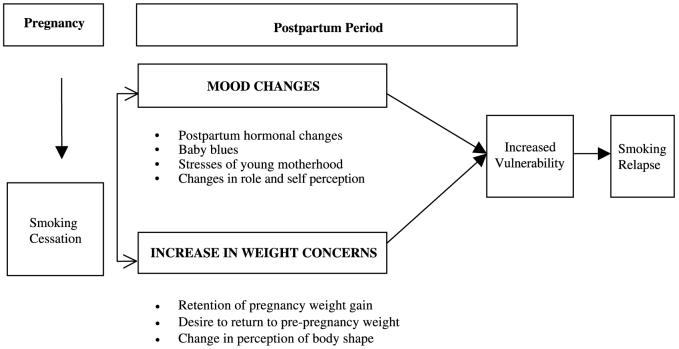
In some cases, examination of the correlates of postpartum smoking has been hampered by short periods of follow-up (Severson et al., 1995) or the lack of biochemically-validated smoking status (McBride and Pirie, 1990). Moreover, as mentioned previously, there has been little assessment of other factors conceptually relevant to the postpartum period that may make this a particularly high-risk time for women smokers to relapse. Specifically, as reviewed below, considerable evidence has suggested that changes in mood and concerns about shape and weight are common during this stage of a woman's life. There also is substantial evidence relating these factors to women's relapse to smoking. Thus, as depicted in Fig. 1, changes in mood and concerns about concerns may relate to women's resumption of smoking in the postpartum period.
Fig. 1.
Model of postpartum smoking relapse
Timing of postpartum smoking interventions
Another potential shortcoming of previous postpartum relapse prevention studies is the point in time at which the intervention has been delivered. With one exception (McBride et al., 1999), previous postpartum relapse prevention efforts have delivered advice about remaining abstinent after delivery during pregnancy. It is possible that the timing of these interventions has minimized their effectiveness, and recent research suggests that relapse prevention interventions delivered during the postpartum period may be more effective than those delivered prenatally.
McBride and colleagues (1999) compared the efficacy of a relapse prevention program delivered during the prenatal period to a prenatal intervention coupled with postnatal phone calls and mailings. At six months postpartum, women who received both the pre- and postpartum intervention were less likely to have relapsed than were those who received only the prenatal intervention. Although the difference in relapse rates between these groups was only marginally significant, survival analyses indicated that women receiving the postpartum contact relapsed less rapidly. In addition, the postpartum intervention used in this study was minimal, and a more intensive or targeted intervention delivered during the high-risk postpartum period might be more likely to decrease the rates of relapse.
Moreover, compared to the earlier stages of pregnancy, the third trimester of pregnancy is associated with increased anxiety (Da Costa et al., 1999). Thus, the salience of relapse prevention and women's interest or ability to utilize information provided to her at this time may be diminished, particularly if she has not experienced urges to return to smoking during the end of pregnancy. Counseling delivered during the postpartum period, when it is likely women may be experiencing urges to smoke, may be more salient. Substantial evidence also has shown the risk of relapse to be greatest during the six months immediately following delivery (McBride and Pirie, 1990; Mullen et al., 1990).
Thus, a relapse prevention program may be most efficacious if it is offered during the postpartum period when it is likely that women are experiencing urges to resume smoking. Moreover, a postpartum relapse prevention program could be designed to address the specific issues that increase a woman's vulnerability to smoking while she is learning to cope with these problems. Below, we review the evidence that two specific areas, concerns about weight and problems with mood, increase women's vulnerability to postpartum smoking.
Concerns about weight and changes in mood relate to postpartum smoking
Weight concerns and women's smoking
The association between smoking cessation and weight gain has been well documented (e.g., Hudmon et al., 1999; Killen et al., 1996; McBride et al., 1996; Perkins et al., 2001). Smoking cessation is associated with weight gain (e.g., Hudmon et al., 1999; Klesges et al., 1997; Streater et al., 1989), and many women endorse the use smoking as a weight control strategy. For example, nearly 40% of young women claim that they smoke specifically to control their weight (Klesges and Klesges, 1988). The issue of smoking and weight gain may be particularly troublesome for women with specific smoking related weight concerns because these weight-concerned women smokers are expressly unwilling to tolerate even modest weight gains (Levine et al., 2001).
Concern about potential weight gain following a quit attempt is more common among women than men (Meyers et al., 1997; Pomerleau and Kurth, 1996; Sorensen and Pechacek, 1987), and this weight concern is associated with cessation failure. Smokers with weight concerns are more likely to drop out of treatment (Mizes et al., 1998) and have poorer cessation outcomes than do smokers who are not concerned about weight gain (Meyers et al., 1997). In addition, evidence suggests that a woman's confidence in her ability to control her weight after quitting relates to higher levels of intention to quit smoking (Secker-Walker et al., 1996), and remaining abstinent from smoking has been associated with increased confidence in preventing weight gain (McBride et al., 1996).
Weight concerns as a factor in postpartum smoking relapse
Not only are weight concerns common during the postpartum period, but there also are several reasons for women to be concerned about weight postpartum. Most women retain a portion of the weight gained during pregnancy in the year after delivery (Keppel and Taffel, 1993), and pregnancy has been related to the development of weight problems among women (Walker, 1995). In addition, maladaptive eating attitudes, dieting behaviors and concerns about shape or weight increase during the postpartum period (Baker et al., 1999; Stein and Fairburn, 1996).
Because concerns about weight are not only common during the postpartum period, but also relate to smoking cessation, it seems likely that weight concerns increase a woman's vulnerability to relapse during the postpartum period. For instance, it is possible that women smokers with weight concerns will struggle with the weight gain of pregnancy more than their peers who are not concerned about weight. This struggle may be particularly intense for women who quit smoking during pregnancy. The combination of a woman's smoking related weight concerns and her thoughts about the relationship between smoking and weight control could precipitate a return to smoking during the postpartum period.
Although the data are limited and the definition of weight concerns has varied across studies, available evidence supports the notion that concerns about weight gain or the use of smoking as a weight control strategy may increase a woman's vulnerability to smoking during the postpartum period. Concerns about weight (McBride and Pirie, 1990; McBride et al., 1992) and having gained more than an average amount of weight during pregnancy (Carmichael et al., 2000) are associated with postpartum smoking relapse. Similarly, the use of snacking as a strategy to cope with smoking urges during pregnancy relates to postpartum relapse (McBride et al., 1992). Moreover, women with greater concerns about shape and weight are more likely than those with fewer weight concerns to endorse the use of smoking as a postpartum weight control strategy (Pomerleau et al., 2000). Thus, considerable theoretical and growing empirical evidence suggest that weight concerns increase women's vulnerability to resume smoking during the postpartum period.
Mood and women's smoking
Mood changes are a second potential smoking relapse trigger with particular relevance to the postpartum period. Research focusing on the relationship between mood and smoking has used numerous conceptualizations of mood, and the lack of discrimination among mood disorders, depressive symptoms and self-reported mood states has complicated comparisons across research studies. For the purpose of this review, mood has been conceptualized as the experience of depressive symptoms and stress, both of which are prevalent during the postpartum period and have been related to women's smoking.
The relationship between mood and smoking has been well documented (Hall et al., 1993). Lifetime rates of major depressive disorder are more common among smokers than nonsmokers (Breslau, 1995). Smokers also endorse more depressive symptoms than nonsmokers do (Lumley et al., 1994). Negative mood and depressive symptoms are associated with relapses to smoking (Ginsberg et al., 1995; Killen et al., 1996; Pomerleau et al., 2001; Shiffman et al., 1996), and recent data suggest that even mild levels of depressive symptomatology relate to a rapid return to smoking, independent of nicotine dependence (Niaura et al., 2001). Not surprisingly, women with higher levels of depressive symptoms are more likely to smoke during pregnancy than are those with less depressive symptomatology (Hoffman and Hatch, 2000). Thus, there is a strong relationship between smoking and mood, which may be exacerbated during the postpartum period for women who have quit smoking during pregnancy.
Mood as a factor in postpartum smoking relapse
The experience of depressive symptoms during the postpartum period is common. Although prevalence rates for clinically significant major depressive disorder during the postpartum are relatively low (3–10%; Cox et al., 1982; Steiner, 1998; Whiffen, 1992), many women experience elevated depressive symptoms or minor depression (Hopkins et al., 1984; Whiffen, 1992). The experience of depressive symptoms following childbirth has been linked to the hormonal shifts following delivery (e.g., Ahokas et al., 2001; Steiner, 1998), family history of psychiatric disorder (Steiner, 1998), depressed mood during pregnancy (e.g., Da Costa et al., 2000) and the experience of negative life events (Swendsen and Mazure, 2000), and it is probable that many factors interact to make the transitional period after childbirth a particularly vulnerable time for women.
It also is widely accepted that new mothers face numerous stressors, and stress both in the pre- and postnatal periods relates to postpartum depressive symptomatology. Ritter and colleagues (2000) found the experience of stressful life events during pregnancy to be related to postpartum depressive symptoms. Others have noted that stressors specific to motherhood (e.g., childcare stressors) as well as general adverse events increase a woman's risk for depressive symptoms following childbirth (Swendsen and Mazure, 2000). Moreover, preliminary data suggest that the experience of stressful life events modestly increases a woman's risk of relapsing to smoking in the postpartum period (Carmichael et al., 2000). Because stress and depressive symptomatology are related to smoking, and because both states are common during the postpartum period, mood is another postpartum-specific relapse trigger.
The interaction of mood and weight concerns may relate to relapse
The interaction of mood and weight concerns may further increase a women's vulnerability to smoking relapse. For example, there is considerable evidence that negative mood relates to overeating. Numerous studies have shown eating disorder symptomatology to be positively associated with depressive symptomatology (Joiner et al., 1995; Killen et al., 1994; Ledoux et al., 1991; Poulakis and Wertheim, 1993). The relationship between negative mood and eating is particularly true among women characterized by high levels dietary restraint, but has also been demonstrated among those with subclinical eating disorder symptomatology (Levine and Marcus, 1997). College females, dichotomized according to their self-reported degrees of habitual dietary restraint, have been shown to increase their food consumption in response to a variety of stressful experiences (Cools et al., 1992; Frost et al., 1982; Heatherton et al., 1991; Herman et al., 1987; Ruderman, 1985; Schotte et al., 1990). Similarly, postpartum weight retention may contribute to negative mood, a well-known trigger of smoking relapse.
Taken together, negative mood may stimulate overeating in vulnerable individuals, and overeating may contribute to weight gain or a failure to lose pregnancy related weight, either of which might further contribute to negative mood. Because both weight and mood independently relate to smoking, it is possible that the interactive effects of weight and mood create a stronger vulnerability to smoking relapse than either factor alone. Thus, the interaction of mood and weight concerns is a third smoking trigger particularly salient during the postpartum period.
Summary
In summary, the majority of women who quit smoking during pregnancy will resume smoking within six months of delivery. Because of the serious health consequences of smoking for mothers, infants and children, preventing postpartum relapse is a critical public health concern. Importantly, evidence suggests that relapse prevention programs are more successful if counseling is continued during the postpartum period (McBride et al., 1999). Thus, identifying potentially modifiable factors related to relapses in the postpartum period may be particularly important to help decrease the number of women smokers. That is, because many women will quit during a pregnancy, preventing postpartum smoking relapse represents a parsimonious way to decrease the public health burden associated with smoking.
As reviewed above, concerns about weight and difficulty managing moods are associated with smoking relapse, and are likely to present considerable challenge to the maintenance of cigarette abstinence during the postpartum period. (see Fig. 1.) Specifically, concerns about mood and weight tend to be elevated during the postpartum period. Both factors also have been related to women's smoking, and preliminary data support the assertion that weight concerns (Carmichael et al., 2000; McBride and Pirie, 1990; Severson et al., 1997) and mood or life stress (Carmichael et al., 2000) relate to postpartum smoking relapse. However, to date, no research has specifically examined the contributions of changes in mood and weight concerns to relapse. Thus, although little is known about the specific correlates of postpartum smoking relapse, we have reasoned that concerns about body weight and negative mood or depressive symptoms are likely to be factors in the resumption of smoking at this time.
Directions for future research
Clearly there is a need for additional research to identify the role that mood and weight concerns play in postpartum smoking relapses. First, a more thorough assessment of the role mood and weight concerns play in relapse is needed. Our research group currently is conducting a study designed to assess the model shown in Fig. 1. We are particularly interested in exploring the ways in which these factors relate to smoking during the postpartum period above and beyond factors that have previously been related to postpartum smoking (i.e., breast feeding, partner smoking, alcohol use), and factors related to smoking in general (e.g., nicotine dependence).
If mood and weight do indeed contribute to postpartum smoking, a second avenue of future research is the design and development of postpartum relapse prevention programs that target postpartum-specific factors. Adjunctive treatments designed to address issues related to women's postcessation weight concerns (Perkins et al., 1997) and mood management (Hall et al., 1994; 1996) have been developed. These and similar treatment programs could be modified and adapted for the prevention of postpartum smoking relapse.
In addition, research testing the dissemination of efficacious treatments for postpartum smoking relapse prevention into traditional medical settings, such as pediatric and obstetric practices, is an important next step. Previous research has examined the effectiveness of training physicians to address smoking in primary care practices (e.g., Manfredi et al., 2000; Windsor et al., 2000). A final avenue for future research on the treatment of postpartum smoking concerns the combination of pharmacotherapy and counseling to help women maintain smoking abstinence after childbirth. The prescription of medications to new mothers who may be breastfeeding is controversial. However, it may be that counseling alone is insufficient to promote continued abstinence among women, particularly those with comorbid mood problems during the postpartum period.
Conclusions
The majority of women who quit smoking during pregnancy will relapse during the postpartum period. Concerns about weight and changes in mood, stress and depressive symptoms are common postpartum issues and may play a role in increasing women's vulnerability to postpartum smoking relapse. Although future work is needed to fully assess the roles of mood, weight concerns and their interaction in the prediction of postpartum smoking, both mood and weight concerns are modifiable factors. Thus, if changes in mood and concerns about weight emerge as postpartum-specific relapse triggers, treatment programs designed to ameliorate women's struggles with weight and mood may increase the percentage of women who maintain smoking abstinence following childbirth, and thus decrease the number of women smokers overall.
References
- Ahokas A, Kaukoranta J, Wahlbeck K, Aito M. Estrogen deficiency in severe postpartum depression: Successful treatment with sublingual physiologic 17B-estradiol: A preliminary study. J Clin Psychiatry. 2001;62:332–336. doi: 10.4088/jcp.v62n0504. [DOI] [PubMed] [Google Scholar]
- Baker CW, Carter AS, Cohen LR, Brownell KD. Eating attitudes and behaviors in pregnancy and postpartum: Global stability versus specific transitions. Ann Behav Med. 1999;21:143–148. doi: 10.1007/BF02908295. [DOI] [PubMed] [Google Scholar]
- Baron JA, LaVecchia C, Levi F. The antiestrogenic effect of cigarette smoking in women. Am J Obstet Gynecol. 1990;162:504–514. doi: 10.1016/0002-9378(90)90420-c. [DOI] [PubMed] [Google Scholar]
- Breslau N. Psychiatric comorbidity of smoking and nicotine. Behav Genet. 1995;25:95–101. doi: 10.1007/BF02196920. [DOI] [PubMed] [Google Scholar]
- Campbell E, Sanson-Fisher R, Walsh R. Smoking status in pregnant women: Assessment of self-report against carbon monoxide. Addict Behav. 2001;26:1–9. doi: 10.1016/s0306-4603(00)00070-8. [DOI] [PubMed] [Google Scholar]
- Carmichael SL, Ahluwalia IB, PRAMS Working Group Correlates of Postpartum Smoking Relapse: Results from the Pregnancy Risk Assessment Monitoring System (PRAMS) Am J Prev Med. 2000;19:193–196. doi: 10.1016/s0749-3797(00)00198-7. [DOI] [PubMed] [Google Scholar]
- Castelao JE, Yuan JM, Skipper PL, Tannenbaum SR, Gago-Dominquez M, Crowder JS, Ross RK, Yu MC. Gender and smoking related bladder cancer risk. J Natl Cancer Inst. 2001;93:538–545. doi: 10.1093/jnci/93.7.538. [DOI] [PubMed] [Google Scholar]
- Castle PE, Wacholder S, Lorincz AT, Scott DR, Sherman ME, Glass AG, Rush BB, Schussler JE, Schiffman M. A prospective study of high-grade cervical neoplasia risk among human papillomavirus-infected women. J Natl Cancer Inst. 2002;94:1406–1414. doi: 10.1093/jnci/94.18.1406. [DOI] [PubMed] [Google Scholar]
- Cools J, Schotte DE, McNally RJ. Emotional arousal and overeating in restrained eaters. J Abnorm Psychol. 1992;101:348–351. doi: 10.1037//0021-843x.101.2.348. [DOI] [PubMed] [Google Scholar]
- Cornelius MD, Day NL. The effects of tobacco use during and after pregnancy on exposed children: Relevance of findings for alcohol research. Alcohol Res Health. 2000;24:242–249. [PMC free article] [PubMed] [Google Scholar]
- Cox JL, Connor Y, Kendell RE. Prospective study of the psychiatric disorders of childbirth. Br J Psychiatry. 1982;140:111–117. doi: 10.1192/bjp.140.2.111. [DOI] [PubMed] [Google Scholar]
- Da Costa DLJ, Drista M, Brender W. Variations in stress levels over the course of pregnancy: Factors associated with elevated hassles, state anxiety and pregnancy-specific stress. J Psychosom Res. 1999;47:609–621. doi: 10.1016/s0022-3999(99)00064-1. [DOI] [PubMed] [Google Scholar]
- Da Costa D, Larouche J, Drista M, Brender W. Psychosocial correlates of prepartum and postpartum depressed mood. J Affect Disord. 2000;59:31–40. doi: 10.1016/s0165-0327(99)00128-7. [DOI] [PubMed] [Google Scholar]
- Dybing E, Sanner T. Passive smoking, sudden infant death syndrome (SIDS) and childhood infections. Hum Exp Toxicol. 1999;18:202–205. doi: 10.1191/096032799678839914. [DOI] [PubMed] [Google Scholar]
- Ebrahim SH, Floyd RL, Merritt RK, Decoufle P, Holtzman D. Trends in pregnancy-related smoking rates in the United States, 1987–1996. JAMA. 2000;283:361–366. doi: 10.1001/jama.283.3.361. [DOI] [PubMed] [Google Scholar]
- Ershoff DH, Mullen DM, Quinn VP. A randomized trial of a serialized self-help smoking cessation program for pregnant women in an HMO. Am J Public Health. 1989;79:182–187. doi: 10.2105/ajph.79.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey JL, Holberg CJ, Aldous MB, Wright AL, Martinez FD, Taussig LM. Passive smoking exposure and otitis media in the first year of life: Group Health Medical Associates. Pediatrics. 1995;95:670–677. [PubMed] [Google Scholar]
- Fingerhut LA, Kleinman JC, Kendrick JS. Smoking before, during, and after pregnancy. Am J Public Health. 1990;80:541–544. doi: 10.2105/ajph.80.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd RL, Rimer BK, Giovino GA, Mullen PD, Sullivan SE. A review of smoking in pregnancy: Effects on pregnancy outcomes and cessation efforts. Annu Rev Public Health. 1993;14:379–411. doi: 10.1146/annurev.pu.14.050193.002115. [DOI] [PubMed] [Google Scholar]
- Frost RO, Goolkasian GA, Ely R, Blanchard FA. Depression, restraint and eating behavior. Behav Res Ther. 1982;20:113–121. doi: 10.1016/0005-7967(82)90111-5. [DOI] [PubMed] [Google Scholar]
- Gielen AC, Windsor R, Faden RR, O'Campo P, Repke J, Davis M. Evaluation of a smoking cessation intervention fro pregnant women in an urban prenatal clinic. Health Ed Res. 1997;12:247–254. doi: 10.1093/her/12.2.247. [DOI] [PubMed] [Google Scholar]
- Ginsberg D, Hall SM, Reus VI, Munoz RF. Mood and depression diagnosis in smoking cessation. Experim Clin Psychopharmacol. 1995;3:389–395. [Google Scholar]
- Hall SM, Munoz RF, Reus VI, Sees KL. Nicotine, negative affect and depression. J Consult Clin Psychol. 1993;61:761–767. doi: 10.1037//0022-006x.61.5.761. [DOI] [PubMed] [Google Scholar]
- Hall SM, Munoz RF, Reus VI. Cognitive-behavioral intervention increases abstinence rates for depressive-history smokers. J Consult Clin Psychol. 1994;62:141–146. doi: 10.1037//0022-006x.62.1.141. [DOI] [PubMed] [Google Scholar]
- Hall SM, Munoz RF, Reus VI, Carol D, Humfleet GL, Hartz DT. Mood management and nicotine gum in smoking treatment: A therapeutic contact and placebo controlled study. J Consult Clin Psychol. 1996;64:1003–1009. doi: 10.1037//0022-006x.64.5.1003. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Herman CP, Polivy J. Effects of physical and ego threat on eating behavior. J Pers Soc Psychol. 1991;60:138–143. doi: 10.1037//0022-3514.60.1.138. [DOI] [PubMed] [Google Scholar]
- Herman CP, Polivy J, Lank CN, Heatherton TF. Anxiety, hunger and eating behavior. J Abnorm Psychol. 1987;96:264–269. doi: 10.1037//0021-843x.96.3.264. [DOI] [PubMed] [Google Scholar]
- Hoffman S, Hatch M. Depressive symptomatology during pregnancy: Evidence for an association with decreased fetal growth in pregnancies of lower social class women. Health Psychol. 2000;19:535–543. [PubMed] [Google Scholar]
- Hopkins J, Marcus MD, Campbell SB. Postpartum depressions: A critical review. Psychol Bull. 1984;95:498–515. [PubMed] [Google Scholar]
- Hudmon KS, Gritz ER, Clayton S, Nisenbaum R. Eating orientation, postcessation weight gain, and continued abstinence among female smokers receiving an unsolicited smoking cessation intervention. Health Psychol. 1999;18:29–36. doi: 10.1037//0278-6133.18.1.29. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Ratner PA, Bottorff JL, Hall W, Dahinten S. Preventing smoking relapse in postpartum women. Nurs Res. 2000;49:44–52. doi: 10.1097/00006199-200001000-00007. [DOI] [PubMed] [Google Scholar]
- Joiner TE, Metalsky GI, Wonderlich SA. Bulimic symptoms and the development of depressive symptoms: The moderating role of attributional style. Cogn Ther Res. 1995;19:651–666. [Google Scholar]
- Kahn RS, Zuckerman B, Bauchner H, Homer CJ, Wise PH. Women's Health After Pregnancy and Child Out comes at age 3 years: a prospective cohort study. Am J Public Health. 2002;92:1312–1318. doi: 10.2105/ajph.92.8.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel KG, Taffel SM. Pregnancy-related weight gain and retention: Implications of the 1990 institute of medicine guidelines. Am J Public Health. 1993;83:1100–1103. doi: 10.2105/ajph.83.8.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen JD, Hayward C, Wilson DM, Taylor CB, Hammer LD, Litt I, Simmonds B, Haydel F. Factors associated with eating disorder symptoms in a community sample of 6th and 7th grade girls. Int J Eat Disord. 1994;15:357–367. doi: 10.1002/eat.2260150406. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Kraemer HC, Varady AN, Davis L, Barbara N. Interactive effects of depression symptoms, nicotine dependence, and weight change on late smoking relapse. J Consult Clin Psychol. 1996;64:1060–1067. doi: 10.1037//0022-006x.64.5.1060. [DOI] [PubMed] [Google Scholar]
- Klesges RC, Klesges LM. Cigarette smoking as a dieting strategy in a university population. Int J Eat Disord. 1988;7:413–419. [Google Scholar]
- Klesges RC, Winders SE, Meyers AW, Eck LH, Ward KD, Hultquist CM, Ray JW, Shadish WR. How much weight gain occurs following smoking cessation? A comparison of weight gain using both continuous and point prevalence abstinence. J Consult Clin Psychol. 1997;65:286–291. doi: 10.1037//0022-006x.65.2.286. [DOI] [PubMed] [Google Scholar]
- Kure EH, Ryberg D, Hewer A, Phillips DH, Skaug V, Baera R, Haugen A. p53 mutations in lung tumors: Relationship to gender and lung DNA adduct levels. Carcinogenesis. 1996;17:2201–2205. doi: 10.1093/carcin/17.10.2201. [DOI] [PubMed] [Google Scholar]
- Langhammer A, Johnson R, Holmen J, Bjermer GL. Cigarette smoking gives more respiratory symptoms among women than among men. J Epidemiol Commun Health. 2000;54:917–922. doi: 10.1136/jech.54.12.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux S, Choquet M, Flament M. Eating disorders among adolescents in an unselected French population. Int J Eat Disord. 1991;10:81–89. [Google Scholar]
- LeLong N, Kaminski M, Saurel-Cubizolles MJ, Bouvier-Colle MH. Post-partum return to smoking among usual smokers who quit during pregnancy. Europ J Publ Health. 2001;11:334–339. doi: 10.1093/eurpub/11.3.334. [DOI] [PubMed] [Google Scholar]
- Levine MD, Marcus MD. Eating behavior following stress in women with and without bulimic symptoms. Ann Behav Med. 1997;19:132–138. doi: 10.1007/BF02883330. [DOI] [PubMed] [Google Scholar]
- Levine MD, Perkins KA, Marcus MD. The characteristics of women smokers concerned about postcessation weight gain. Addict Behav. 2001;26:749–756. doi: 10.1016/s0306-4603(00)00156-8. [DOI] [PubMed] [Google Scholar]
- Lumley MA, Downey K, Stetner L, Wehmer F, Pomerleau OF. Alexithymia and negative affect: Relationship to cigarette smoking, nicotine dependence, and smoking cessation. Psychother Psychosom. 1994;6:156–162. doi: 10.1159/000288884. [DOI] [PubMed] [Google Scholar]
- Manfredi C, Crittenden KS, Cho YI, Engler J, Warnecke R. Minimal smoking cessation interventions in prenatal, family planning, and well-child health clinics. Am J Publ Health. 2000;90:423–427. doi: 10.2105/ajph.90.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews TJ. Smoking during pregnancy in the 1990s. Natl Vital Stat Rep. 2001;49:1–15. [PubMed] [Google Scholar]
- Maughan B, Taylor C, Taylor A, Butler N, Bynner J. Pregnancy smoking and childhood conduct problems: A casual association? J Child Psychiatry. 2001;42:1021–1028. doi: 10.1111/1469-7610.00800. [DOI] [PubMed] [Google Scholar]
- McBride CM, Pirie PL. Postpartum smoking relapse. Addict Behav. 1990;15:165–168. doi: 10.1016/0306-4603(90)90020-x. [DOI] [PubMed] [Google Scholar]
- McBride CM, Pirie PL, Curry SJ. Postpartum relapse to smoking: A prospective study. Health Educ Res. 1992;7:381–390. doi: 10.1093/her/7.3.381. [DOI] [PubMed] [Google Scholar]
- McBride CM, French SA, Pirie PL, Jeffery RW. Changes over time in weight concerns among women smokers engaged in the cessation process. Ann Behav Med. 1996;18:273–279. doi: 10.1007/BF02895289. [DOI] [PubMed] [Google Scholar]
- McBride CM, Curry SJ, Lando HA, Pirie PL, Grothaus LC, Nelson JC. Prevention of relapse in women who quit smoking during pregnancy. Am J Public Health. 1999;89:706–711. doi: 10.2105/ajph.89.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers AW, Klesges RC, Winders SE, Ward KD, Peterson BA, Eck LH. Are weight concerns predictive of smoking cessation? A prospective analysis. J Consult Clin Psychol. 1997;65:448–452. doi: 10.1037//0022-006x.65.3.448. [DOI] [PubMed] [Google Scholar]
- Mizes JS, Sloan DM, Segraves K, Spring B, Pingitore R, Kristeller J. The influence of weight-related variables on smoking cessation. Behavior Therapy. 1998;29:371–385. [Google Scholar]
- Mullen PD, Quinn VP, Ershoff DH. Maintenance of nonsmoking postpartum by women who stopped smoking during pregnancy. Am J Public Health. 1990;80:992–994. doi: 10.2105/ajph.80.8.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen PD, DiClemente C, Carbonari J, Sockrider M, Nicol L, Richardson MA, Taylor W. Project panda maintenance of pre-natal smoking abstinence postpartum at 6 weeks and 3, 6, and 12 months. Ann Behav Med. 1997;19:S130. [Google Scholar]
- Niaura R, Britt DM, Shadel WG, Goldstein M, Abrams D, Brown R. Symptoms of depression and survival experience among three samples of smokers trying to quit. Psychol Addict Behav. 2001;15:13–17. doi: 10.1037/0893-164x.15.1.13. [DOI] [PubMed] [Google Scholar]
- Owen L, McNeill A. Salvia cotinine as indicator of cigarette smoking in pregnant women. Addiction. 2001;96:1001–1006. doi: 10.1046/j.1360-0443.2001.96710019.x. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Levine MD, Marcus MD, Shiffman S. Addressing women's concerns about weight gain due to smoking cessation. J Subst Abuse Treat. 1997;14:173–182. doi: 10.1016/s0740-5472(96)00158-4. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Marcus MD, Levine MD, D'Amico D, Miller A, Broge M, Ashcom J, Shiffman S. Cognitive-behavioral therapy to reduce weight concerns improves smoking cessation outcome in weight-concerned women. J Consult Clin Psychol. 2001;69:604–613. [PubMed] [Google Scholar]
- Pomerleau CS, Kurth CL. Willingness of female smokers to tolerate postcessation weight gain. J Subst Abuse. 1996;8:371–378. doi: 10.1016/s0899-3289(96)90215-1. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Brouwer RJN, Jones LT. Weight concerns in women smokers during pregnancy and postpartum. Addict Behav. 2000;25:759–767. doi: 10.1016/s0306-4603(00)00086-1. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Brouwer RJN, Pomerleau OF. Emergence of depression during early abstinence in depressed and non-depressed women smokers. J Addict Dis. 2001;20:73–80. doi: 10.1300/J069v20n01_07. [DOI] [PubMed] [Google Scholar]
- Poulakis Z, Wertheim EH. Relationships among dysfunctional cognitions, depressive symptoms, and bulimic tendencies. Cogn Therapy Res. 1993;17:549–559. [Google Scholar]
- Prescott E, Hippe M, Schrohr P, de Heis H, Vestbo J. Smoking and risk of myocardial infarction in women and men: Longitudinal population study. Br Med J. 1998;316:1043–1047. doi: 10.1136/bmj.316.7137.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner PA, Johnson JL, Bottorff JL, Dahinten S, Hall W. Twelve-month follow-up of a smoking relapse prevention intervention for postpartum women. Addict Behav. 2000;25:81–92. doi: 10.1016/s0306-4603(99)00033-7. [DOI] [PubMed] [Google Scholar]
- Ritter C, Hobfall SE, Lavin J, Cameron RP, Hulsizer MR. Stress, psychosocial resources, and depressive symptomatology during pregnancy in low-income, inner-city women. Health Psychol. 2000;19:576–585. doi: 10.1037//0278-6133.19.6.576. [DOI] [PubMed] [Google Scholar]
- Ruderman AJ. Dysphoric mood and overeating: A test of restraint theory's disinhibition hypothesis. J Abnorm Psychol. 1985;23:151–156. doi: 10.1037//0021-843x.94.1.78. [DOI] [PubMed] [Google Scholar]
- Schotte DE, Cools J, McNally RJ. Film-induced negative affect triggers overeating in restrained eaters. J Abnorm Psychol. 1990;99:317–320. doi: 10.1037//0021-843x.99.3.317. [DOI] [PubMed] [Google Scholar]
- Secker-Walker RH, Lepage SS, Solomon LJ, Goodwin GD, Flynn BS, Mead PB, Skelly JM. Smoking relapse prevention counseling during prenatal and early postnatal care. Am J Prev Med. 1995;11:86–93. [PubMed] [Google Scholar]
- Secker-Walker RH, Flynn BS, Solomon LJ, Vacek PM. Helping women quit smoking: Baseline observations for a community health education project. Am J Prev Med. 1996;12:367–377. [PubMed] [Google Scholar]
- Secker-Walker RH, Solomon LJ, Flynn BS, Skelly JM, Mead PB. Smoking relapse prevention during pregnancy: A trial of coordinated advice from physicians and individual counseling. Am J Prev Med. 1998;15:25–31. doi: 10.1016/s0749-3797(98)00029-4. [DOI] [PubMed] [Google Scholar]
- Severson HH, Andrews JA, Lichtenstein E, Wall M, Zoref L. Predictors of smoking during and after pregnancy: A survey of mothers of newborns. Prev Med. 1995;24:23–28. doi: 10.1006/pmed.1995.1004. [DOI] [PubMed] [Google Scholar]
- Severson HH, Andrews JA, Lichtenstein E, Wall M, Akers L. Reducing maternal smoking and relapse: Long-term evaluation of a pediatric intervention. Prev Med. 1997;26:120–130. doi: 10.1006/pmed.1996.9983. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: Within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Sorensen G, Pechacek T. Attitudes toward smoking cessation among men and women. J Behav Med. 1987;10:129–137. doi: 10.1007/BF00846421. [DOI] [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification Biochemical verification of tobacco use and cessation. Nicotine Tobacco Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Stein A, Fairburn CG. Eating habits and attitudes in the postpartum period. Psychosom Med. 1996;58:321–325. doi: 10.1097/00006842-199607000-00004. [DOI] [PubMed] [Google Scholar]
- Steiner M. Perinatal mood disorders: Position paper. Psychopharmacol Bull. 1998;34:301–306. [PubMed] [Google Scholar]
- Stotts AL, DiClemente CC, Carbonari JP, Mullen PD. Postpartum return to smoking: Staging a “suspended” behavior. Health Psychol. 2000;19:324–332. doi: 10.1037//0278-6133.19.4.324. [DOI] [PubMed] [Google Scholar]
- Streater JA, Sargent RG, Ward DS. A study of factors associated with weight change in women who attempt smoking cessation. Addict Behav. 1989;14:523–530. doi: 10.1016/0306-4603(89)90072-5. [DOI] [PubMed] [Google Scholar]
- Swendsen JD, Mazure CM. Life stress as a risk factor for postpartum depression: Current research and methodological issues. Clinical Psychology: Science Practice. 2000;7:17–31. [Google Scholar]
- U.S. Department of Health and Human Services Women and smoking: A report of the Surgeon General. 2001 [Google Scholar]
- Valanis B, Lichtenstein E, Mullooly JP, Labuhn K, Brody K, Severson HH, Stevens N. Maternal smoking cessation and relapse prevention during health care visits. Am J Prev Med. 2001;20:1–8. doi: 10.1016/s0749-3797(00)00266-x. [DOI] [PubMed] [Google Scholar]
- Van't Hoff SM, Wall MA, Dowler DW, Stark MJ. Randomised controlled trial of a postpartum relapse prevention intervention. Tobacco Control. 2000;9(Suppl III):64–66. doi: 10.1136/tc.9.suppl_3.iii64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LO. Weight gain after childbirth: A women's health concern. Ann Behav Med. 1995;17:132–141. doi: 10.1007/BF02895062. [DOI] [PubMed] [Google Scholar]
- Wall MA, Severson HH, Andrews JA, Lichtenstein E, Zoref L. Pediatric office-based smoking intervention: Impact on maternal smoking and relapse. Pediatrics. 1995;96:622–628. [PubMed] [Google Scholar]
- Whiffen VE. Is postpartum depression a distinct diagnosis. Clin Psychol Rev. 1992;12:485–508. [Google Scholar]
- Windsor RA, Woodby LL, Miller TM, Hardin JM, Crawford MA, DiClemente CC. Effectiveness of agency for health care policy and research clinical practice guideline and patient education methods for pregnant smokers in Medicaid maternity care. Am J Obstet Gynecol. 2000;182:68–75. doi: 10.1016/s0002-9378(00)70492-3. [DOI] [PubMed] [Google Scholar]
- Zang EA, Wynder EL. Differences in lung cancer risk between men and women: Examination of the evidence. J Natl Cancer Inst. 1996;88:183–192. doi: 10.1093/jnci/88.3-4.183. [DOI] [PubMed] [Google Scholar]