Abstract
The past relationship between global temperature and levels of biological diversity is of increasing concern due to anthropogenic climate warming. However, no consistent link between these variables has yet been demonstrated. We analysed the fossil record for the last 520 Myr against estimates of low latitude sea surface temperature for the same period. We found that global biodiversity (the richness of families and genera) is related to temperature and has been relatively low during warm ‘greenhouse’ phases, while during the same phases extinction and origination rates of taxonomic lineages have been relatively high. These findings are consistent for terrestrial and marine environments and are robust to a number of alternative assumptions and potential biases. Our results provide the first clear evidence that global climate may explain substantial variation in the fossil record in a simple and consistent manner. Our findings may have implications for extinction and biodiversity change under future climate warming.
Keywords: fossil record, global biodiversity, global climate change, global temperature, macroevolution, mass extinction
1. Introduction
The possibility that anthropogenic climate change could cause much extinction (Thomas et al. 2004; Lovejoy & Hannah 2005; Pounds et al. 2006; Botkin et al. 2007) has placed new emphasis on studies of the relationship between global temperature and levels of biological diversity (Crowley & North 1988; Alroy et al. 2000; Culver & Rawson 2000; Barnosky et al. 2003, 2004; Wilf et al. 2003; Prothero 2004; Gibbs et al. 2006). Past analyses of this relationship have shown that past climate variation is sometimes associated with variation in biodiversity and taxonomic rate, but effects vary considerably, and no consistent relationship has emerged (see above refs). However, most studies have been confined to relatively short geological periods, limited geographical extents and/or few taxonomic groups. In recent years, palaeoclimate datasets have been developed (Veizer et al. 2000; Royer et al. 2004) that, together with biodiversity compilations from the fossil record (Benton 1993; Sepkoski 2002), allows this relationship to be tackled at a much broader scale than has been possible before.
The explanation of patterns in global biodiversity and taxonomic rates in the fossil record is one of the major challenges in evolutionary biology. Previous research has focused mainly on identifying (i) main trends and their consistency with alternative models of cladogenesis (Levinton 1979; Sepkoski 1984; Benton 1995, 1997; Courtillot & Gaudemer 1996) and (ii) potential causes of periods of major biodiversity turnover known as mass extinction events (Raup & Sepkoski 1982; Crowley & North 1988; Erwin 1990; Wignall & Twitchett 1996; Pope et al. 1998; Sheehan 2001; Joachimski & Buggisch 2002; White 2002; Benton & Twitchett 2003; Wignall 2005; Twitchett 2006). Only rarely have studies attempted to find statistical associations between the major global patterns and environmental variables throughout the Phanerozoic (Rothman 2001; Cornette et al. 2002).
We tested for the first time whether a major climate measure, temperature, is statistically linked with variation in global biodiversity and taxonomic rates over the Phanerozoic. Our results show, robustly, that biodiversity and evolutionary processes are indeed linked to global temperature in a consistent manner over the Phanerozoic fossil record.
2. Material and methods
The temperature data are based on low latitude sea surface oxygen isotope ratios corrected for variation in seawater pH (Royer et al. 2004). These data are built on earlier estimates (Veizer et al. 2000), providing a better fit to first-order tests such as known glacial periods (see Veizer et al. 2000; Royer et al. 2004). The major features of these data, in terms of ‘icehouse’ and ‘greenhouse’ climatic phases in Earth history, are widely supported and non-contentious (Huber et al. 2000). We conducted a sensitivity analysis on the temperature data, using predictions generated by two alternative assumptions about seawater calcium (Royer et al. 2004), corresponding to the upper and lower orange bands of fig. 4 in Royer et al. (2004).
Data on the distribution of families across geological strata (Benton 1993) represent the most recent compilation of global diversity covering both land and sea. The data were compiled under two different assumptions about the timing of fossil appearances for those families where dating was not to stage level (e.g. epoch); the ‘maximum’ assumption assumes that the lifespan of each family was from the first stage of the interval of first appearance until last stage of the interval of last appearance. The ‘minimum’ assumption is that the lifespan of a family was from the last stage of the interval of first appearance until the first stage of the interval of last appearance (Benton 1993, 1995). Data on marine genera of animals and protists (Sepkoski 2002) were compiled at stage resolution to provide maximum taxonomic inclusivity and greatest stratum comparability with Benton (1993). Three of the four major datasets we have used (the temperature, CO2 and global family datasets) apply the same time scale (Harland et al. 1990). Therefore to maximize comparability among the different datasets and to reduce total rescaling error, because strata used in different scales are not always exactly equivalent, we rescaled the more recent genus level marine dataset to the same time scale. Taxonomic rates were recalculated using the new assumed time scale.
We included estimated atmospheric CO2 concentrations (Berner & Kothavala 2001) as an explanatory variable in some analyses because previous analyses have shown that this may correlate with both taxonomic richness and rates (Rothman 2001; Cornette et al. 2002), and because CO2 levels and temperature are often good predictors of each other (Royer et al. 2004). Estimates of CO2 levels over the Phanerozoic in recent years have often been discordant (Berner & Kothavala 2001; Rothman 2002). We used those from the GEOCARB III model primarily because those data were assumed in deriving the temperature estimates we used (Royer et al. 2004). The data have also been used in previous studies on macroevolution (Cornette et al. 2002), show good convergence with other models that track carbon exchange, match well with other proxies (Royer et al. 2004) and correlate well with temperature (Royer et al. 2004), so are likely to provide a rigorous test of the effects of temperature itself.
We first used traditional measures of taxonomic diversity and rates as our response variables; total richness and the per-taxon rate (Foote 2000a). However, such measures may be susceptible to a number of biases that may obscure the true biological signal (Foote 2000a). We therefore used taxonomic rate measures that are independent of singletons: the standing richness of boundary crossing taxa (Foote 2000b), as well as the estimated per capita origination rate ‘p’ and extinction rate ‘q’ (Foote 2000a). Such measures are potentially more robust to variation in interval length and preservation rate, at least as long as this variation is small. Second, we included several control variables in our analysis to represent variation in preservation rate: numbers of sedimentary formations at epoch level were taken from columns 3 and 4 of appendix 2 of Peters & Foote (2001). Stage level data for marine formations (same original source) were obtained from Peters & Foote (2002). Data from the Correlation of Stratigraphic Units of North America used in Peters (2005) were obtained courtesy of the author. Finally, data on estimated marine genus preservation frequency in Foote (2005; from the pulsed/pulsed model) were obtained courtesy of the author. All these datasets were rescaled, as above for the Sepkoski data. The temperature and CO2 data consist of estimates at 10 Myr intervals and to provide richness and rate data at the same intervals we used the diversity and taxonomic rates of the geological interval into which each 10 Myr point fell.
Time series (see electronic supplementary material for dataset and explanatory text) were transformed (log or square root), as appropriate, and detrended to remove long-term patterns (see table 1 in the electronic supplementary material). The detrending methodology was to fit linear and cubic polynomials, and splines increasing in flexibility to follow the patterns in the data (i.e. of increasing degrees of freedom, d.f.; Fewster et al. 2000; see figure 1 and table 1 in the electronic supplementary material). The specific detrender used was chosen to: make the series stationary; reduce the influence of outliers; make the distribution symmetrical; and maximize the signal-to-noise ratio. Low d.f. smoothers fit the trend and as the d.f. increase the smoothers pick up more of the shorter-term patterns in the data. Comparison between the fits, using autocorrelation or spectral analysis (see figure 2 in the electronic supplementary material), typically shows a pattern dominated by the trend (i.e. autocorrelation decays away very slowly), whereas very high d.f. smoothers take out all but noise around the pattern (so there are no patterns in the autocorrelation function). The appropriate smoother is one that maximizes the underlying signal. In the majority of cases, the detrender was a 5 d.f. spline (see figure 2 and table 2 in the electronic supplementary material). Detrended time series were then mean standardized.
Pearson correlation was used between the detrended diversity series and the temperature series. Generalized linear models were fitted with the diversity series as the dependent variable. The latter method allowed statistical controlling for covariates, and therefore tested whether the covariates (e.g. preservation rates or CO2) could explain the patterns in the series. As points in a time series are serially autocorrelated, all significance was established using bootstrapping of the appropriate statistic (the correlation, the difference between lagged and unlagged correlation). We tested for associations between diversity or taxonomic rates and temperature during the same time step. Autocorrelation and spectral analysis sometimes suggested lagged associations, diversity or taxonomic rates lagging behind temperature, so we also tested for such responses, normally using a lag of 10 Myr between the time series.
3. Results
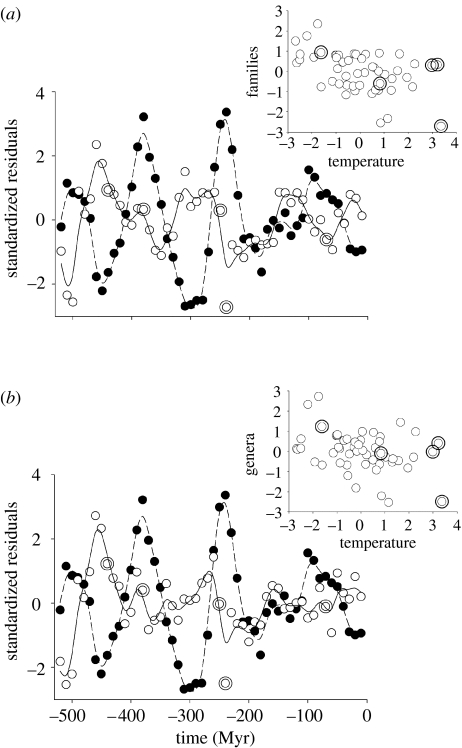
The standing diversity of fossil families was significantly negatively associated with temperature using both maximum and minimum dating assumptions (table 1; figure 1). The correlation was significantly stronger at a lag of one time step (10 Myr) using maximum dating estimates, but not when using minimum dates (table 1). When the data were split into marine and terrestrial families, all relationships remained negative, but only some attained statistical significance. For example, for marine families, the correlation was also significant, though lagged results were not significantly stronger than unlagged; and for marine genera, the relationship was also negative, but not significant. For terrestrial families, all trends remained negative but only one correlation was significant: that using the maximum dating assumption, and at a 10 Myr lag (with a much shorter fossil record the statistical power is weaker).
Table 1.
Correlations between temperature and standing diversity, origination rate and extinction rate. (Numbers are Pearson correlation coefficients for (transformed, detrended, mean standardized) temperature against the column variable, *p<0.05. Coefficients for unlagged data are always given. If coefficients for lagged data were stronger, these are also given (italics if coefficient is significantly stronger than unlagged). Details of data transformation, splines used to calculate residuals+CIs of r are given in the electronic supplementary material, table 2.)
| standing diversity | origination rate | extinction rate | ||||||
|---|---|---|---|---|---|---|---|---|
| taxon | dating assumption | time lag (Myr) | total diversity | boundary crossers | per-taxon rate | estimated per capita rate, p | per-taxon rate | estimated per capita rate, q |
| all families | maximum | 0 | −0.481* | −0.456* | 0.428* | 0.388* | ||
| 10 | −0.512* | −0.497* | 0.462* | |||||
| marine families | 0 | −0.376* | 0.529* | 0.476* | 0.519* | |||
| 10 | 0.529* | |||||||
| terrestrial families | 0 | −0.166 | −0.191 | 0.292 | 0.269 | 0.387* | ||
| 10 | −0.427* | 0.375 | 0.349 | |||||
| 40 | −0.361* | |||||||
| all families | minimum | 0 | −0.474* | 0.460* | 0.350* | 0.461* | 0.493* | |
| 10 | 0.357* | |||||||
| marine families | 0 | −0.414* | −0.439* | 0.484* | 0.505* | 0.505* | ||
| 10 | −0.497* | 0.488* | 0.515* | |||||
| terrestrial families | 0 | −0.009 | 0.199 | 0.429* | 0.461* | |||
| 10 | −0.182 | |||||||
| marine genera | n.a. | 0 | −0.190 | −0.339* | 0.563* | 0.531* | 0.492* | 0.398* |
| 10 | −0.361* | |||||||
Figure 1.
Taxonomic diversity against temperature. Time series of temperature and (a) standing diversity (number of families) using the maximum dating assumption and (b) standing diversity of boundary-crossing marine animal genera. Diversity and temperature were transformed, detrended and mean standardized. Closed circles and dashed lines represent temperature (°C) and open circles and continuous lines diversity. Large double open symbols represent periods of mass extinction, defined as the five largest positive extinction residuals (see text, figure 3). Curves are fitted using a 25 d.f. spline. Insets show the negative association between diversity and temperature residuals across the time series.
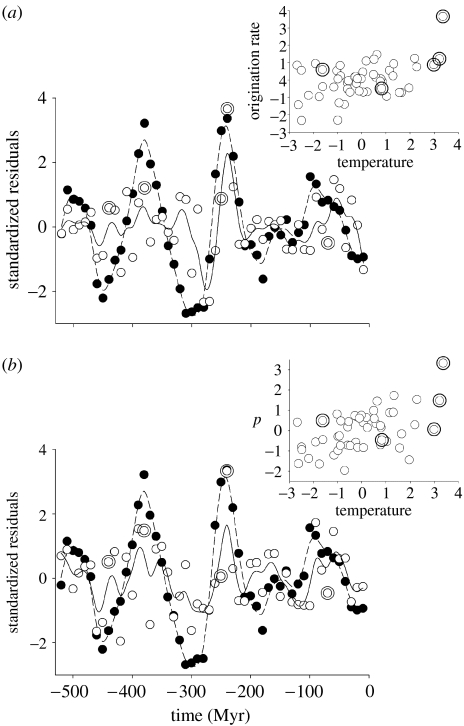
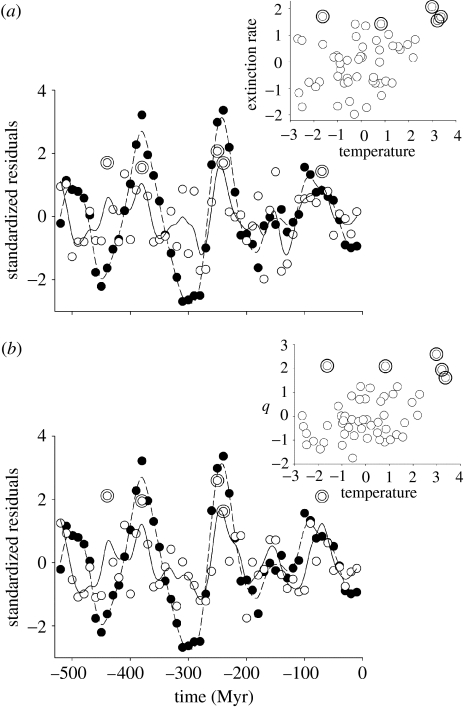
In contrast, the per-taxon origination rates were all positively associated with temperature (table 1; figure 2), attaining significance for all families, marine families and marine genera. Again, some correlations were significantly stronger following a 10 Myr lag. Per-taxon extinction rates were also significantly positively correlated with temperature for all groups (table 1; figure 3), and in no case were lagged correlations stronger than unlagged. Thus, high rates of extinction occurred at the same time as, or preceded, periods of reduced overall taxonomic diversity and increased origination of taxonomic lineages (Sepkoski 1998; Foote 2000b, 2005).
Figure 2.
Origination rate against temperature. Time series of temperature and (a) the per-taxon rate of origination (Myr−1) for all families using the maximum dating assumption and (b) the estimated per capita rate, p, of origination (Myr−1) for marine animal genera. Rates and temperature were transformed, detrended and mean standardized. Closed circles and dashed lines represent temperature (°C) and open circles and continuous lines origination rates. Large double open symbols represent periods of mass extinction, defined as the five largest positive extinction residuals (see text, figure 3). Curves are fitted using a 25 d.f. spline. Insets show the positive association between origination rate and temperature residuals across the time series.
Figure 3.
Extinction rate against temperature. Time series of temperature and (a) the per-taxon rate of extinction for families (Myr−1) using the maximum dating assumption and (b) the estimated per capita rate, q, of extinction (Myr−1) for marine animal genera. Rates and temperature were transformed, detrended and mean standardized. Closed circles and dashed lines represent temperature and open circles and continuous lines extinction rates. Large double open symbols represent periods of mass extinction, defined as the five largest positive extinction residuals (in order of decreasing age: end Ordovician; Late Devonian; end Permian; Early Triassic, end Cretaceous). Curves are fitted using a 25 d.f. spline. Insets show the positive association between extinction rate and temperature residuals across the time series.
When we used measures of standing diversity, origination and extinction rate that are independent of singletons (Foote 2000a), the strength and significance of correlations were generally unaffected (table 1: boundary crossers, p, q). However, marine genus diversity became significant (figure 1), suggesting that the significant results based on traditional measures are robust to some potential biases, but also that some signal is obscured by the incorporation of singletons.
In general, associations remain in the same direction throughout the time series, though during some time intervals they differ or are weak. For example, in the Mid–Late Jurassic (ca 180–150 Ma), both temperature and diversity residuals rise, giving rise to a positive association (figure 1). Extinction and temperature residuals are rather poorly associated during the Cambrian and Ordovician (ca 520–440 Ma), and extinction residuals are sometimes positive during the icehouse Carboniferous phase (ca 350–280 Ma; figure 3).
When atmospheric CO2 concentrations were included as an explanatory variable in our analyses, temperature always remained significant, and CO2 was normally not significant (table 1 in the electronic supplementary material). CO2 was significant for both marine genus origination and extinction rate, and in the latter case was a stronger predictor than temperature. Overall, temperature was the better predictor of diversity and taxonomic rates.
The results were robust to different temperature estimates based on different assumptions about seawater calcium concentrations (Royer et al. 2004; table 1 in the electronic supplementary material). Incorporating a number of measures of (or proxies for) preservation probability as explanatory variables (Peters & Foote 2001, 2002; Foote 2005; Peters 2005) showed that the results were robust: although preservation probability variables were normally significantly positively associated with measures of diversity and taxonomic rates, temperature still explained a significant proportion of the variance in diversity or taxonomic rates in the same direction as before (table 1 in the electronic supplementary material).
4. Discussion
Variation in global temperature has previously been implicated in several major features of the fossil record, most notably mass extinction events (see Wignall 2005; Twitchett 2006) such as the end-Ordovician mass extinction, during a period of glaciation (Crowley & North 1988; Sheehan 2001), and the end-Permian mass extinction, during an extremely warm climatic phase (Erwin 1990; White 2002; Benton & Twitchett 2003). However, we show here that, against a background trend of increasing diversity over time, global climate is consistently correlated not only with variation in extinction rates across the Phanerozoic, but with biodiversity and origination rates as well. We emphasize that our results relate only to a second-order effect in that we have detrended the variables in question prior to analysis. This has consequences for interpreting our results since the effects of global temperature are superimposed on long-term trends in increasing diversity and decreasing extinction and origination rates (see below), which doubtless have independent causes (e.g. Benton 1997).
In the current rapid transition from an icehouse to a greenhouse world (Huber et al. 2000; Lovejoy & Hannah 2005; IPCC 2007), Earth history may help us to estimate future effects on biodiversity (Culver & Rawson 2000). Prima facie, our results from the fossil record endorse those of ecological models (Thomas et al. 2004; Botkin et al. 2007), which demonstrate that expected future warming will adversely affect biodiversity. However, several qualifications are necessary.
A first qualification is that our results relate to the effects of residuals from the long-term trend. An increase in global temperature may therefore cause an increase in extinction rate but not necessarily an absolute decrease in biodiversity because the underlying trend is for biodiversity to increase over time.
A second qualification is that the coarse time scale of our data does not allow us to make short-term predictions, although short-term effects also cannot be excluded. Related to this, in predictive models relating extinction to climate change, it is the changes in climate rather than the future climates themselves that are generally held responsible (Thomas et al. 2004; Lovejoy & Hannah 2005; Botkin et al. 2007; Williams et al. 2007). Our results suggest that long-term average temperatures may exert a separate effect, independent of the rate of temperature change, and thus may be related to an entirely different mechanism (see below). Furthermore, the associations we have shown are only moderate in strength (correlations ≤0.5), and time intervals are apparent when associations can be reversed.
Finally, although we have shown an association between temperature and both biodiversity and taxonomic rates, this association may not be causative. Deducing causation from correlation is, of course, difficult. The lags shown in some of our analyses suggest that temperature is affecting biodiversity and evolutionary rates, but well-known links between organisms and geophysical processes suggest we should not yet rule out the opposite direction of causation (Rothman 2001). The periodic cycle of taxonomic richness and rates seen here (ca 140 Myr, figures 1–3) is also potentially associated with cosmic ray flux, the age of meteorites and possibly sea-level changes (Rohde & Muller 2005). However, the age of meteorites is likely to be linked causally to cosmic ray flux (Rohde & Muller 2005), and the latter (Shaviv & Veizer 2003) and sea-level changes (Hallam & Wignall 1999) are likely to be linked to temperature. Clearly, many of these variables are likely to exert independent or correlated effects, and both our response and explanatory variables could also be responding independently to other variables not considered here.
Previous work at the scale of the Phanerozoic has suggested associations between atmospheric CO2 concentrations and taxonomic richness and rates (Rothman 2001; Cornette et al. 2002). However, those studies were more taxonomically restricted, and solely used traditional richness and rate measures that are known to be susceptible to biases in the fossil record (Foote 2000a). Because it still remains significant in some of our analyses, our results suggest that CO2 may still play some direct role on biodiversity and taxonomic rates independent of temperature, though for most of our analyses temperature is more important.
Despite the above provisos, our results demand that we speculate on causative links between temperature and both biodiversity and taxonomic rates. Previous work has suggested that extinction rates primarily drive changes in Phanerozoic diversity (Foote 2000b) and that origination rates rise following mass extinctions (see Sepkoski 1998). Thus it is plausible that the associations between temperature and both biodiversity and origination are primarily driven by changes in extinction rates with temperature. The lagged associations between temperature and origination rates, but not extinction rates, are consistent with this interpretation.
The five largest positive residuals for extinction in our time series correspond to previously identified mass extinctions (Raup & Sepkoski 1982; Benton 1995) in the end Ordovician, Late Devonian, end Permian, Early Triassic (a continuance of the former) and end Cretaceous (figure 3). Of these, four correspond well with peaks in global temperature in the time series (figure 3). Thus, our analyses suggest that these mass extinctions can be viewed as part of a wider trend seen across the fossil record as a whole. A number of recent studies have suggested a link between periods of rapid global warming, perhaps driven by large igneous province eruptions, and marine crises that often accompany extinction events (Wignall 2005; Twitchett 2006). It is plausible that there is an interaction between the long-term average global temperature and the onset of such marine crises, such that the effects of rapid global warming on marine systems are exacerbated in a world that is already in a greenhouse state, increasing the probability of oceanic anoxia or breakdowns in oceanic circulation. However, the associations shown in this paper apply to terrestrial as well as marine systems, and it is currently unclear how such marine crises might affect terrestrial systems (Wignall 2005).
The risk of future extinction through rapid global warming is primarily expected to occur through mismatches between the climates to which organisms are adapted in their current range and the future distributions of those climates (Thomas et al. 2004; Botkin et al. 2007; Williams et al. 2007), a mechanism that has doubtless also been relevant in the past (Twitchett 2006). Because taxa adapted to more tropical environments may be more vulnerable to short-term changes in climate in this way (Stanley 1986; Joachimski & Buggisch 2002; Williams et al. 2007), it is plausible that high long-term global temperatures may increase the general vulnerability of species to rapid climate change, and this may also explain the associations we find.
In conclusion, we have discovered a second-order long-term association between global temperature and both biodiversity and taxonomic rates, and show that whether Earth climate was in an icehouse or greenhouse phase explains considerable variation in the Phanerozoic fossil record. Prima facie, our results suggest that future global warming may be detrimental to biodiversity. However, the mechanisms underlying the association are still unclear, and only when they become clearer we will be in a position to comment confidently on the implications for future climate change.
Acknowledgments
We thank Barbara Anderson for help with data compilation; Michael Foote, Shanan Peters and Dana Royer for providing data; and John Lawton, Chris Thomas and four anonymous referees for their comments.
Supplementary Material
This document defines the column variables in the supplementary dataset, and answers frequently asked questions about our analyses
This Excel spreadsheet contains the 10 Myr interval time series on which our analyses were based, allowing other researchers to repeat or evaluate our analyses
References
- Alroy J, Koch P.L, Zachos J.C. Global climate change and North American mammalian evolution. Paleobiology. 2000;26(Suppl.):259–288. [Google Scholar]
- Barnosky A.D, Hadly E.A, Bell C.J. Mammalian response to global warming on varied temporal scales. J. Mammal. 2003;84:354–368. doi:10.1644/1545-1542(2003)084<0354:MRTGWO>2.0.CO;2 [Google Scholar]
- Barnosky A.D, Bell C.J, Emslie S.D, Goodwin H.T, Mead J.I, Repenning C.A, Scott E, Shabel A.B. Exceptional record of Mid-Pleistocene vertebrates helps differentiate climatic from anthropogenic ecosystem perturbations. Proc. Natl Acad. Sci. USA. 2004;101:9297–9302. doi: 10.1073/pnas.0402592101. doi:10.1073/pnas.0402592101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton M.J, editor. The fossil record 2. Chapman and Hall; London, UK: 1993. [Google Scholar]
- Benton M.J. Diversification and extinction in the history of life. Science. 1995;268:52–58. doi: 10.1126/science.7701342. doi:10.1126/science.7701342 [DOI] [PubMed] [Google Scholar]
- Benton M.J. Models for the diversification of life. Trends Ecol. Evol. 1997;12:490–495. doi: 10.1016/s0169-5347(97)84410-2. doi:10.1016/S0169-5347(97)84410-2 [DOI] [PubMed] [Google Scholar]
- Benton M.J, Twitchett R.J. How to kill (almost) all life: the end-Permian extinction event. Trends Ecol. Evol. 2003;18:358–365. doi:10.1016/S0169-5347(03)00093-4 [Google Scholar]
- Berner R.A, Kothavala Z. GEOCARB III: a revised model of atmospheric CO2 over Phanerozoic time. Am. J. Sci. 2001;301:182–204. doi:10.2475/ajs.301.2.182 [Google Scholar]
- Botkin D.B, et al. Forecasting effects of global warming on biodiversity. Bioscience. 2007;57:227–236. doi:10.1641/B570306 [Google Scholar]
- Cornette J.L, Lieberman B.S, Goldstein R.H. Documenting a significant relationship between macroevolutionary origination rates and Phanerozoic pCO2 levels. Proc. Natl Acad. Sci. USA. 2002;99:7832–7835. doi: 10.1073/pnas.122225499. doi:10.1073/pnas.122225499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtillot V, Gaudemer Y. Effects of mass extinctions on biodiversity. Nature. 1996;381:146–148. doi:10.1038/381146a0 [Google Scholar]
- Crowley T.J, North G.R. Abrupt climate change and extinction events in earth history. Science. 1988;240:996–1002. doi: 10.1126/science.240.4855.996. doi:10.1126/science.240.4855.996 [DOI] [PubMed] [Google Scholar]
- Culver S.J, Rawson P.F, editors. Biotic response to global change: the last 145 million years. Cambridge University Press; Cambridge, UK: 2000. p. 501. [Google Scholar]
- Erwin D.H. The end-Permian mass extinction. Annu. Rev. Ecol. Syst. 1990;21:69–91. doi:10.1146/annurev.es.21.110190.000441 [Google Scholar]
- Fewster R.M, Buckland S.T, Siriwardena G.M, Baillie S.R, Wilson J.D. Analysis of population trends for farmland birds using generalized additive models. Ecology. 2000;81:1970–1984. [Google Scholar]
- Foote M. Origination and extinction components of taxonomic diversity: general problems. Paleobiology. 2000a;26(Suppl.):74–102. [Google Scholar]
- Foote M. Origination and extinction components of taxonomic diversity: Paleozoic and post-Paleozoic dynamics. Paleobiology. 2000b;26:578–605. doi:10.1666/0094-8373(2000)026<0578:OAECOT>2.0.CO;2 [Google Scholar]
- Foote M. Pulsed origination and extinction in the marine realm. Paleobiology. 2005;31:6–20. doi:10.1666/0094-8373(2005)031<0006:POAEIT>2.0.CO;2 [Google Scholar]
- Gibbs S.J, Bown P.R, Sessa J.A, Bralower T.J, Wilson P.A. Nannoplankton extinction and origination across the Paleocene–Eocene thermal maximum. Science. 2006;314:1770–1773. doi: 10.1126/science.1133902. doi:10.1126/science.1133902 [DOI] [PubMed] [Google Scholar]
- Hallam A, Wignall P.B. Mass extinctions and sea-level change. Earth Sci. Rev. 1999;48:217–250. doi:10.1016/S0012-8252(99)00055-0 [Google Scholar]
- Harland W.B, Armstrong R.L, Cox A.V, Craig L.E, Smith A.G, Smith D.G. Cambridge University Press; Cambridge, UK: 1990. A geologic time scale. [Google Scholar]
- Huber B.T, MacLeod K.G, Wing S.L, editors. Warm climates in Earth history. Cambridge University Press; Cambridge, UK: 2000. [Google Scholar]
- Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt K.B, Tignor M, Miller H.L, editors. IPCC. Climate change 2007: the physical science basis. Contribution of Working Group I to the fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press; Cambridge, UK: 2007. p. 18. [Google Scholar]
- Joachimski M.M, Buggisch W. Conodont Apatite δ18O signatures indicate climatic cooling as a trigger of the Late Devonian (F–F) mass extinction. Geology. 2002;30:711–714. doi:10.1130/0091-7613(2002)030<0711:CAOSIC>2.0.CO;2 [Google Scholar]
- Levinton J.S. A theory of diversity equilibrium and morphological evolution. Science. 1979;204:335–336. doi: 10.1126/science.204.4390.335. doi:10.1126/science.204.4390.335 [DOI] [PubMed] [Google Scholar]
- Lovejoy T.E, Hannah L, editors. Climate change and biodiversity. Yale University Press; New Haven, CT: 2005. p. 440. [Google Scholar]
- Peters S.E. Geologic constraints on the macroevolutionary history of marine animals. Proc. Natl Acad. Sci. USA. 2005;102:1236–1331. doi: 10.1073/pnas.0502616102. doi:10.1073/pnas.0502616102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S.E, Foote M. Biodiversity in the Phanerozoic: a reinterpretation. Paleobiology. 2001;27:583–601. doi:10.1666/0094-8373(2001)027<0583:BITPAR>2.0.CO;2 [Google Scholar]
- Peters S.E, Foote M. Determinants of extinction in the fossil record. Nature. 2002;416:420–424. doi: 10.1038/416420a. doi:10.1038/416420a [DOI] [PubMed] [Google Scholar]
- Pope K.O, D'Hondt S.L, Marshall C.R. Meteorite impact and the mass extinction of species at the Cretaceous/Tertiary boundary. Proc. Natl Acad. Sci. USA. 1998;95:11 028–11 029. doi: 10.1073/pnas.95.19.11028. doi:10.1073/pnas.95.19.11028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pounds J.A, et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature. 2006;439:161–167. doi: 10.1038/nature04246. doi:10.1038/nature04246 [DOI] [PubMed] [Google Scholar]
- Prothero D.R. Did impacts, volcanic eruptions, or climatic change affect mammalian evolution? Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004;214:283–294. doi:10.1016/j.palaeo.2004.04.010 [Google Scholar]
- Raup D, Sepkoski J. Mass extinctions in the marine fossil record. Science. 1982;215:1501–1503. doi: 10.1126/science.215.4539.1501. doi:10.1126/science.215.4539.1501 [DOI] [PubMed] [Google Scholar]
- Rohde R.A, Muller R.A. Cycles in fossil diversity. Nature. 2005;434:208–210. doi: 10.1038/nature03339. doi:10.1038/nature03339 [DOI] [PubMed] [Google Scholar]
- Rothman D.H. Global biodiversity and the ancient carbon cycle. Proc. Natl Acad. Sci. USA. 2001;98:4305–4310. doi: 10.1073/pnas.071047798. doi:10.1073/pnas.071047798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman D.H. Atmospheric carbon dioxide levels for the last 500 million years. Proc. Natl Acad. Sci. USA. 2002;99:4167–4171. doi: 10.1073/pnas.022055499. doi:10.1073/pnas.022055499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer D.L, Berner R.A, Montañez I.P, Tibor N.J, Beerling D.J. CO2 as a primary driver of Phanerozoic climate. GSA Today. 2004;14:4–10. doi:10.1130/1052-5173(2004)014<4:CAAPDO>2.0.CO;2 [Google Scholar]
- Sepkoski J.J., Jr A kinetic model of Phanerozoic taxonomic diversity: III. Post-Paleozoic marine families and mass extinctions. Paleobiology. 1984;10:246–267. [Google Scholar]
- Sepkoski J.J., Jr Rates of speciation in the fossil record. Phil. Trans. R. Soc. B. 1998;353:315–326. doi: 10.1098/rstb.1998.0212. doi:10.1098/rstb.1998.0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepkoski J.J., Jr A compendium of fossil marine animal genera. Bull. Am. Paleontol. 2002;363:1–560. [Google Scholar]
- Shaviv N.J, Veizer J. Celestial driver of Phanerozoic climate? GSA Today. 2003;13:4–10. doi:10.1130/1052-5173(2003)013<0004:CDOPC>2.0.CO;2 [Google Scholar]
- Sheehan P.M. The Late Ordovician mass extinction. Annu. Rev. Earth Plan. Sci. 2001;29:331–364. doi:10.1146/annurev.earth.29.1.331 [Google Scholar]
- Stanley S.M. Scientific American Library; New York, NY: 1986. Extinction. [Google Scholar]
- Thomas C.D, et al. Extinction risk from climate change. Nature. 2004;427:145–148. doi: 10.1038/nature02121. doi:10.1038/nature02121 [DOI] [PubMed] [Google Scholar]
- Twitchett R.J. The palaeoclimatology, palaeoecology, and palaeoenvironmental analysis of mass extinction events. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006;232:190–213. doi:10.1016/j.palaeo.2005.05.019 [Google Scholar]
- Veizer J, Godderis Y, François L.M. Evidence for decoupling of atmospheric CO2 and global climate during the Phanerozoic eon. Nature. 2000;408:698–701. doi: 10.1038/35047044. doi:10.1038/35047044 [DOI] [PubMed] [Google Scholar]
- White R.V. Earth's biggest ‘whodunnit’: unravelling the clues in the case of the end-Permian mass extinction. Phil. Trans. R. Soc. A. 2002;360:2963–2985. doi: 10.1098/rsta.2002.1097. doi:10.1098/rsta.2002.1097 [DOI] [PubMed] [Google Scholar]
- Wignall P. The link between large igneous province eruptions and mass extinctions. Elements. 2005;1:293–297. [Google Scholar]
- Wignall P.B, Twitchett R.J. Oceanic anoxia and the end-Permian mass extinction. Science. 1996;272:1155–1158. doi: 10.1126/science.272.5265.1155. doi:10.1126/science.272.5265.1155 [DOI] [PubMed] [Google Scholar]
- Wilf P, Johnson K.R, Huber B.T. Correlated terrestrial and marine evidence for global climate changes before mass extinction at the Cretaceous–Paleogene boundary. Proc. Natl Acad. Sci. USA. 2003;100:599–604. doi: 10.1073/pnas.0234701100. doi:10.1073/pnas.0234701100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.W, Jackson S.T, Kutzbach J.E. Projected distributions of novel and disappearing climates by 2100 AD. Proc. Natl Acad. Sci. USA. 2007;104:5738–5742. doi: 10.1073/pnas.0606292104. doi:10.1073/pnas.0606292104 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This document defines the column variables in the supplementary dataset, and answers frequently asked questions about our analyses
This Excel spreadsheet contains the 10 Myr interval time series on which our analyses were based, allowing other researchers to repeat or evaluate our analyses